Apoptosis is a form of programmed cell death, specifically a regulated cell death process, characterized primarily by nuclear pyknosis, cell shrinkage, membrane blebbing, and DNA fragmentation. Furthermore, caspase family proteases and other key apoptotic regulators play pivotal roles in apoptosis research. Three principal pathways mediate apoptosis: the intrinsic apoptotic pathway, the extrinsic apoptotic pathway, and the endoplasmic reticulum (ER) stress-induced apoptotic pathway.
The intrinsic pathway is activated by oligomerization of the B-cell lymphoma-2 (BCL-2) family proteins BAK and BAX. BAK/BAX oligomers form pores in the mitochondrial outer membrane, leading to the release of cytochrome c into the cytosol. Activation of BAK/BAX is regulated by proapoptotic (e.g., BAD and BID) or antiapoptotic (e.g., BCL-2) BCL-2 family proteins. Cytochrome c binds to Apaf-1, which recruits procaspase-9, forming the apoptosome. In the apoptosome, caspase-9 is activated by autoproteolytic cleavage, initiating the caspase-processing cascade.
The extrinsic pathway is activated by engagement of membrane receptors such as Tumor necrosis factor (TNF) receptor 1 (TNFR1), death receptors, or Toll-Like Receptors (TLRs). These proteins induce the formation of signaling complexes involving TNFR1-associated death domain protein (TRADD) or Fas-associated death domain protein (FADD), receptor-interacting serine/threonine protein kinase 1 (RIPK1) and procaspase-8. Ubiquitylation of RIPK1 by cellular inhibitors of apoptosis (cIAPs) stabilizes the complex and induces the activation of the transcription factor NFκB. FLIP, also present in the DISC, limits caspase-8 activity while promoting cell survival, cell proliferation, and the production of proinflammatory cytokines. Imbalances in this pathway, such as those imposed by cellular stress, allow the activation of caspase-8 and caspase-10, which in turn triggers the caspase activation cascade.
Once active, executioner caspases (i.e., caspase-2, -6, -8 and -10) bring about programmed apoptotic death. Apoptotic cells release messengers in the form of nucleosomal structures, shed receptors, anti-inflammatory metabolites or molecules packaged in apoptotic extracellular vesicles (ApoEVs). Phosphatidylserine (PS) molecules exposed on the outer surface of the plasma membrane function as “eat me” signals for phagocytes.
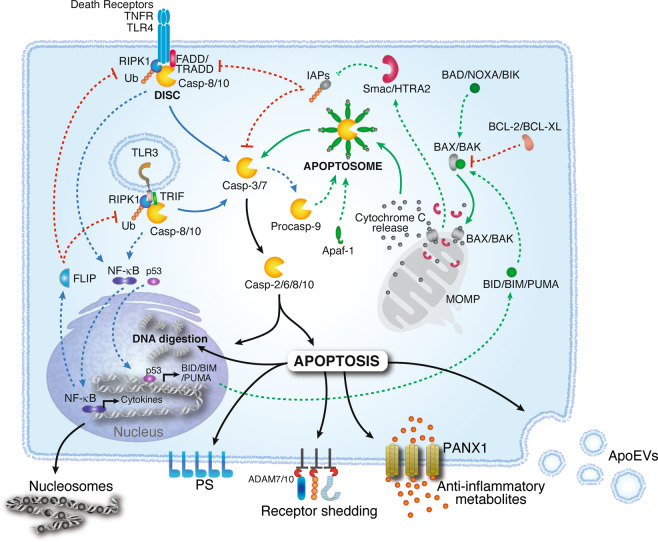
Fig 1. Apoptotic pathways and regulation(Cell Mol Immunol.2021 May;18(5):1106-1121.)
Endoplasmic Reticulum Stress-Induced Apoptotic Pathway: This represents a relatively novel regulatory mechanism for apoptosis. When cells undergo ER stress due to various factors, they initiate the unfolded protein response (UPR). The UPR is activated by three ER stress sensors: inositol-requiring enzyme 1 (IRE1), protein kinase R-like endoplasmic reticulum kinase (PERK), and activating transcription factor 6 (ATF6). IRE1 interacts with the adaptor protein TNF receptor-associated factor 2 (TRAF2), leading to activation of c-Jun N-terminal kinase (JNK). Activated JNK promotes the expression of the pro-apoptotic protein BCL-2-associated death promoter (BAD), thereby inducing apoptosis. Conversely, PERK facilitates the phosphorylation of eukaryotic translation initiation factor 2α (eIF2α) and enhances the translation of activating transcription factor 4 (ATF4). ATF4 upregulates the expression of the ER stress protein C/EBP homologous protein (CHOP). CHOP promotes apoptosis by increasing the expression of pro-apoptotic proteins BCL-2-associated X protein (BAX) and BCL-2 antagonist/killer (BAK).
Apoptosis is an indispensable physiological mechanism for tissue formation during embryogenesis. Indeed, the fine-tuned regulation of cell fate during embryonic development critically depends on mechanisms involving RIPK1 and caspase-8. Additionally, apoptosis serves as an essential tool for the immune system to combat infections and eliminate cells harboring irreparable DNA damage. Dysregulation of apoptosis occurs in numerous pathological conditions, including autoimmune disorders, neurodegenerative diseases, and cancer, contributing to their pathogenesis. Consequently, understanding how apoptosis influences these biological processes may lead to therapeutic advances, ultimately benefiting human health.
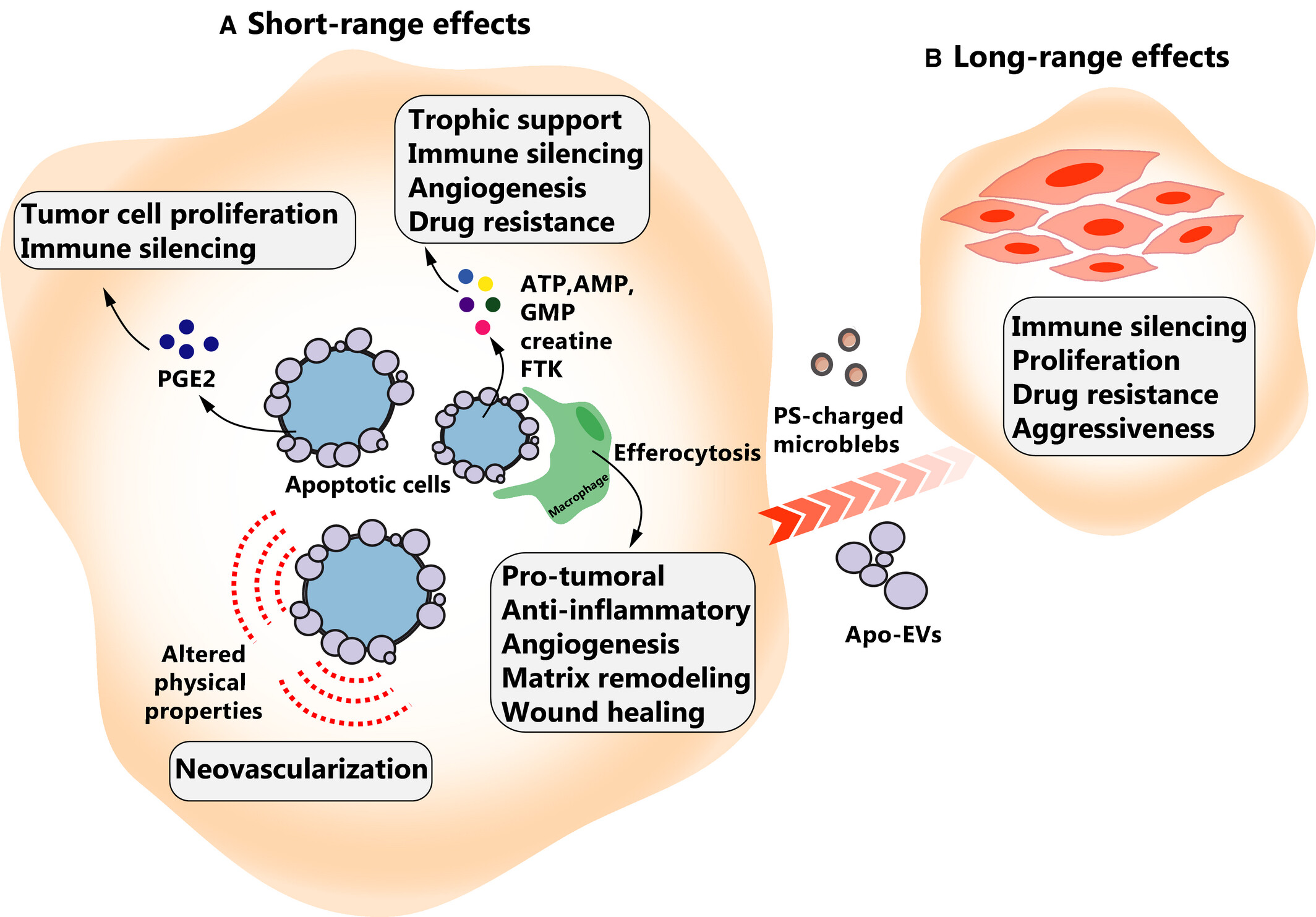
Fig 2. Pro‐oncogenic effects of apoptotic cells(FEBS J.2021 Aug;288(15):4445-4463.)
Reference
1. Castillo Ferrer C, Berthenet K, Ichim G. Apoptosis – Fueling the oncogenic fire. The FEBS Journal. 2020;288(15):4445-4463.
2. Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cellular & Molecular Immunology. 2021;18(5):1106-1121.
AntibodySystem provides apoptosis-related proteins and antibodies, delivering more tools and solutions for apoptosis research.
Recombinant Protein
|
Catalog |
Product Name |
|
YHE35901 |
Recombinant Human CASP3/Caspase-3 Protein, N-His |
|
YHG90201 |
Recombinant Human CASP8 Protein, N-His |
|
YMG90201 |
Recombinant Mouse CASP8 Protein, N-His |
|
YHF11001 |
Recombinant Human CASP9/Caspase 9 Protein, N-His-SUMO |
|
YMG13201 |
Recombinant Mouse BAX Protein, N-His |
|
YHG13201 |
Recombinant Human BAX Protein, N-His |
|
YMG13202 |
Recombinant Mouse BAX Protein, N-His |
|
YHC81001 |
Recombinant Human BCL2 Protein, C-His |
|
YHA27801 |
Recombinant Human APAF1 Protein, N-His |
|
YMJ41101 |
Recombinant Mouse NLRP3 Protein, N-His |
|
YHH14501 |
Recombinant Human TRADD Protein, N-His |
Antibody
|
Catalog |
Product Name |
|
RHE35909 |
Anti-CASP3/Caspase-3 Antibody (R3K78) |
|
RHE35910 |
Anti-CASP3/Caspase-3 Antibody (R3K79) |
|
RHE35901 |
Anti-Human CASP3/Caspase-3 Nanobody (SAA1223) |
|
RHE35903 |
Anti-Human CASP3 Nanobody (VHH2) |
|
RHG13210 |
Anti-BAX Antibody (R3R53) |
|
RHG13211 |
Anti-BAX Antibody (R3R54) |
|
RHG13212 |
Anti-BAX Antibody (R3R55) |
|
RHG13202 |
Anti-Human BAX Nanobody (SAA1222) |
|
RHG13201 |
Anti-Human BAX Antibody (6A7) |
|
RHG13204 |
Anti-Human BAX Antibody (3C10) |
|
RHC81001 |
Anti-Human BCL2 Nanobody (SAA1206) |
|
RHC81005 |
Anti-BCL2 Antibody (R3C07) |
|
RHA27801 |
Anti-APAF1 Antibody (R1B07) |
|
RHA27803 |
Anti-APAF1 Antibody (R2T83) |
|
RHD34001 |
Anti-Human CD120a/TNFRSF1A/TNFR1 Nanobody (SAA1242) |
|
PHD34002 |
Anti-Human CD120a/TNFRSF1A/TNFR1 Polyclonal Antibody |
|
RHD34003 |
Anti-CD120a/TNFRSF1A/TNFR1 Antibody (R3F34) |
|
RHH14501 |
Anti-TRADD Antibody (R3T86) |
|
PHH14501 |
Anti-TRADD Polyclonal Antibody |
|
PHG42701 |
Anti-FADD Polyclonal Antibody |
