Intervertebral disc degeneration (IVDD), a prevalent degenerative spinal disorder, is the leading cause of low back pain. Its incidence rises sharply with population aging, imposing not only severe pain, reduced quality of life, and disability on patients, but also an enormous economic burden on healthcare systems. The core pathological mechanisms include mechanical overload–induced annulus fibrosus (AF) rupture triggering nucleus pulposus (NP) herniation and nerve compression, as well as intradiscal oxidative stress (ROS) accumulation driving cell apoptosis, senescence, inflammation, and extracellular matrix (ECM) metabolic imbalance. Current conservative or surgical treatments are limited by high recurrence rates and lack targeted AF repair. The avascular nature of the disc restricts drug delivery, and repeated local injections may even accelerate degeneration. Therefore, developing a strategy that simultaneously targets oxidative stress and ECM imbalance is urgently needed.
Figure 1. Schematic illustration of intervertebral disc degeneration
To address these issues, a study published in Advanced Functional Materials titled "Photothermal Hydrogel with Mn₃O₄ Nanoparticles Alleviates Intervertebral Disc Degeneration by Scavenging ROS and Regulating Extracellular Matrix Metabolism" developed an injectable photothermal hydrogel, Mn3O4@ChS-HA. This system comprises dopamine-grafted oxidized chondroitin sulfate (dop-OChS), adipic dihydrazide-modified hyaluronic acid (ADH-HA), and Mn3O4 nanoparticles to repair annulus fibrosus (AF) defects. The hydrogel mimics the native intervertebral disc microenvironment, exhibiting exceptional mechanical properties and strong adhesion to AF lesion sites while enabling long-term retention of Mn3O4 nanoparticles. Critically, Mn3O4 nanoparticles exhibit catalytic properties analogous to superoxide dismutase, catalase, and glutathione peroxidase, efficiently scavenging ROS and modulating ECM metabolism.

In this study, mitochondrial DNA leakage induced by oxidative stress was detected using Anti-dsDNA Antibody (3E10#) (Catalog RGK24020) from AntibodySystem via immunofluorescence .
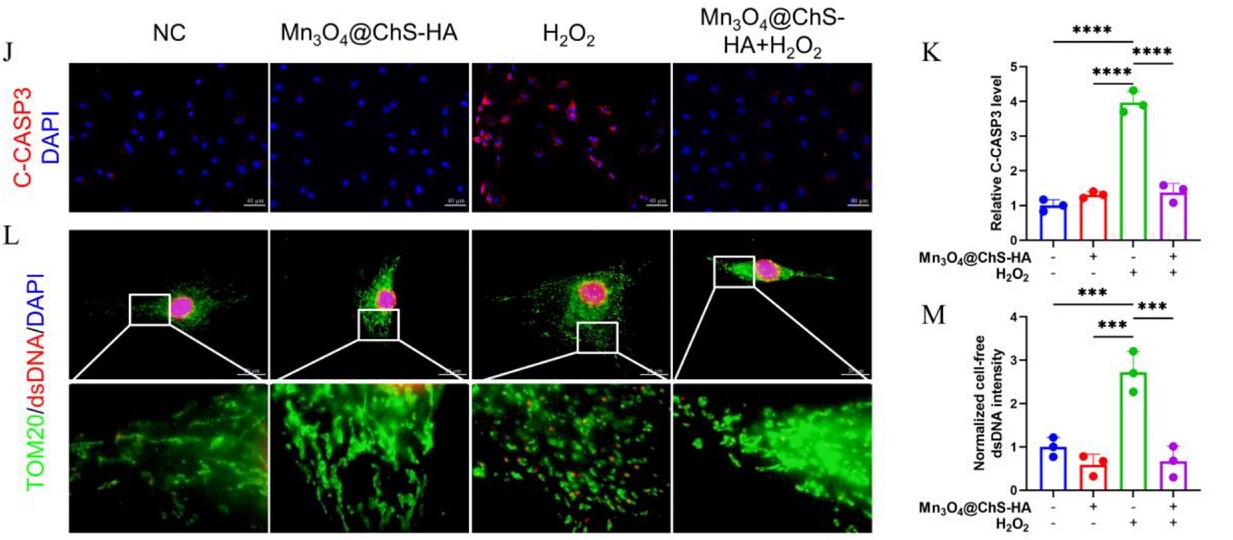
Results demonstrated that H2O2 stimulation significantly increased cytosolic dsDNA levels (red fluorescence), indicating mtDNA leakage and mitochondrial fission. The Mn₃O₄@ChS-HA hydrogel treatment group exhibited markedly reduced cytosolic dsDNA, confirming its capacity to preserve mitochondrial integrity.
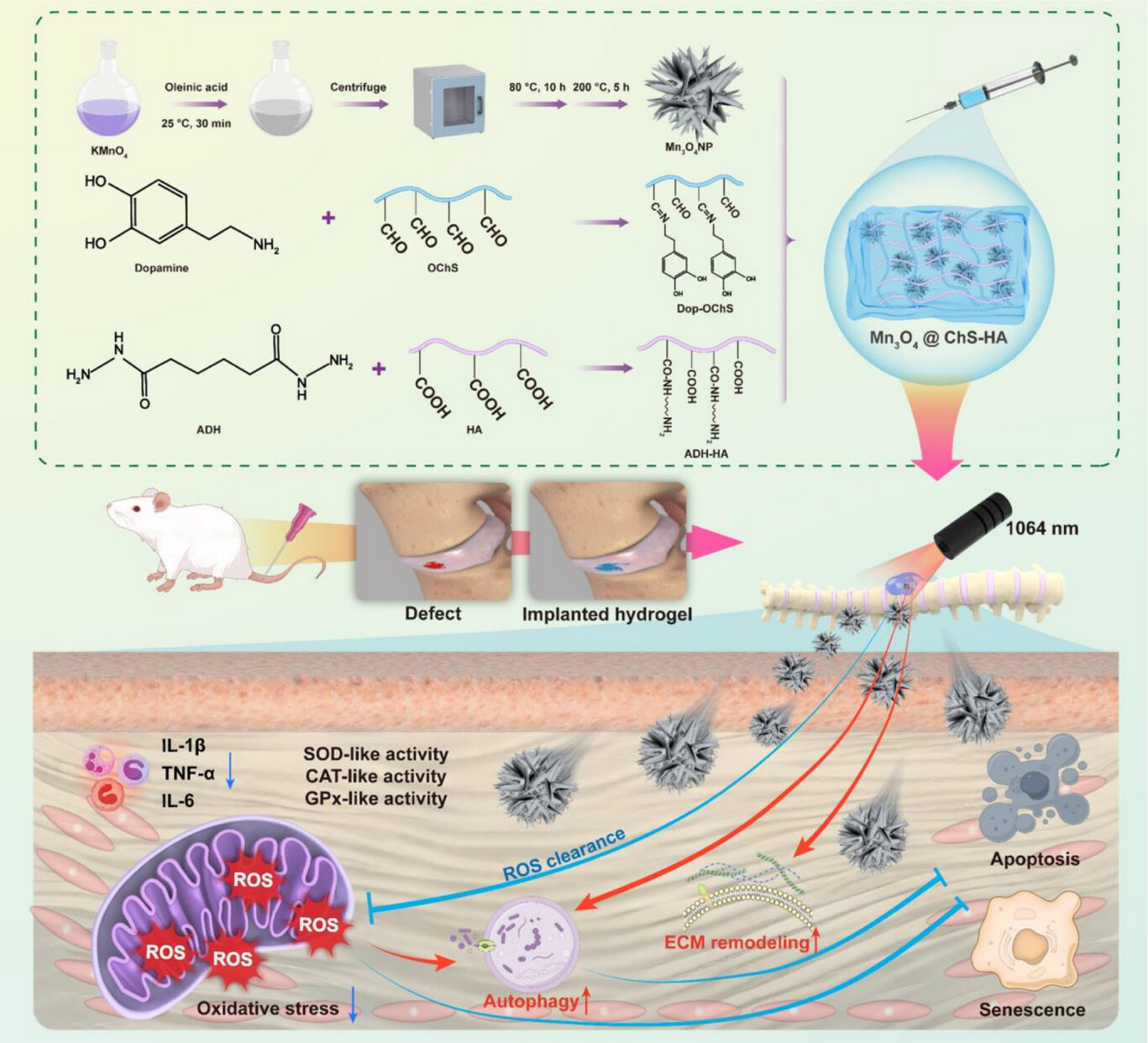
Figure 2. Schematic illustration of the preparation and mechanism of the multifunctional photothermal hydrogel loaded with Mn3O4 NPs for IVDD treatment
Key Findings
Experimental data demonstrate that Mn3O4 NPs simultaneously mimic SOD, CAT, and GPx activities, boosting H2O2 decomposition efficiency by 80%. At the cellular level, this triple-enzyme mimetic activity achieves robust ROS clearance and markedly restores mitochondrial function. Mechanistically, the study reveals a cascade: “mitochondrial ROS removal → membrane potential stabilization → apoptosis inhibition,” offering a novel intervention paradigm for oxidative stress–driven degenerative diseases.
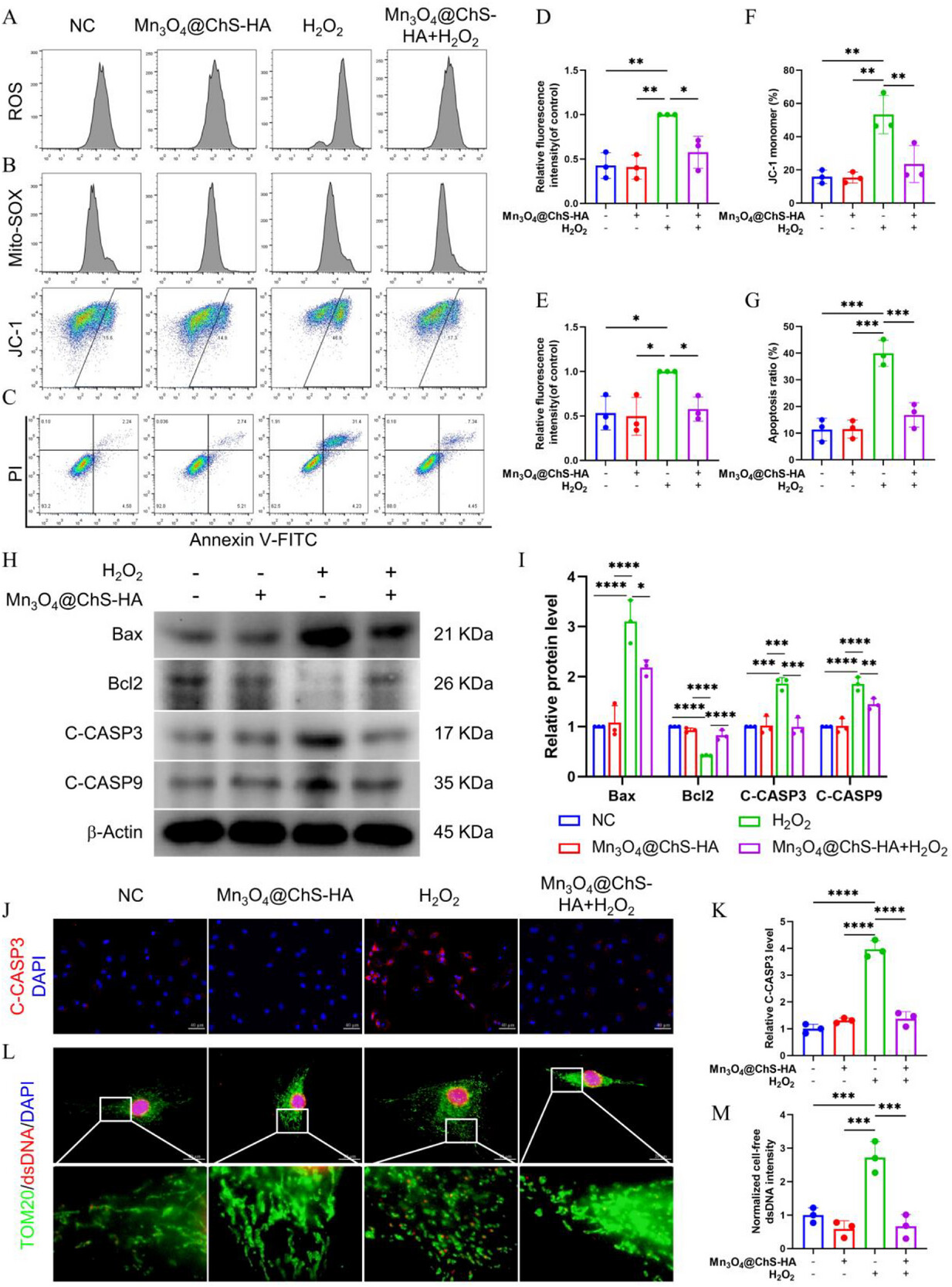
Figure 3. Resistance of Mn3O4@ChS-HA against oxidative stress and AFCs apoptosis
Following Mn3O4@ChS-HA treatment, SA-β-Gal⁺ senescent cells decreased by 70%, while autophagy marker LC3B fluorescence intensity increased 2.1-fold and autophagy initiator BECN1 expression was up-regulated. This indicates that ROS removal directly activates autophagic flux, which in turn clears damaged mitochondria, forming a positive feedback loop of “antioxidation → autophagy enhancement → anti-aging.” Moreover, autophagy activation simultaneously restrains inflammatory cytokine release, challenging the traditional view that senescence and inflammation are separately regulated.
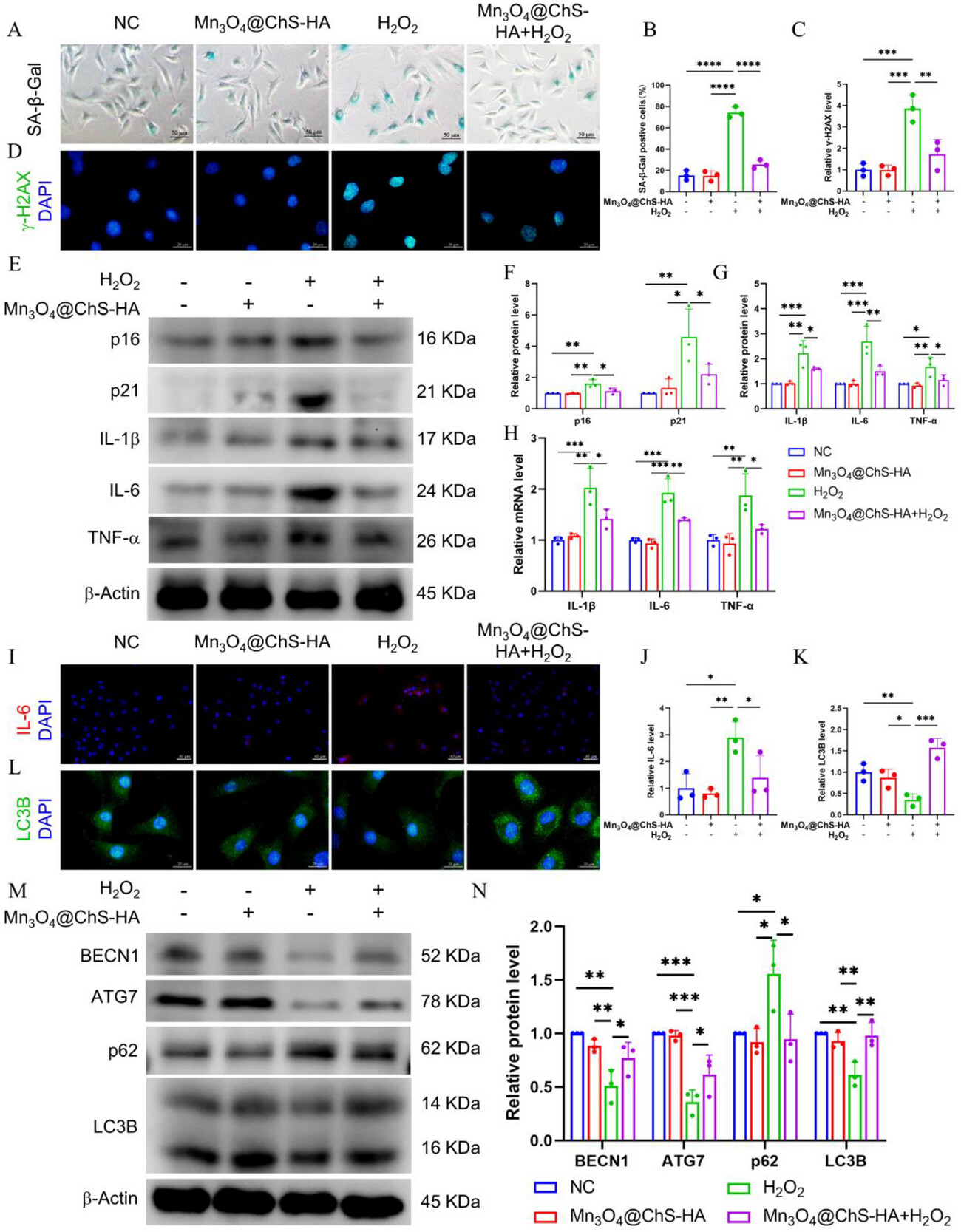
Figure 4. Inhibition of senescence and inflammation, and promotion of autophagy in AFCs by Mn3O4@ChS-HA
At the molecular level, Mn3O4@ChS-HA restored Aggrecan and COL2A1 expression to 190% of normal levels and reduced the matrix-degrading enzymes MMP13/ADAMTS5 by 50%.
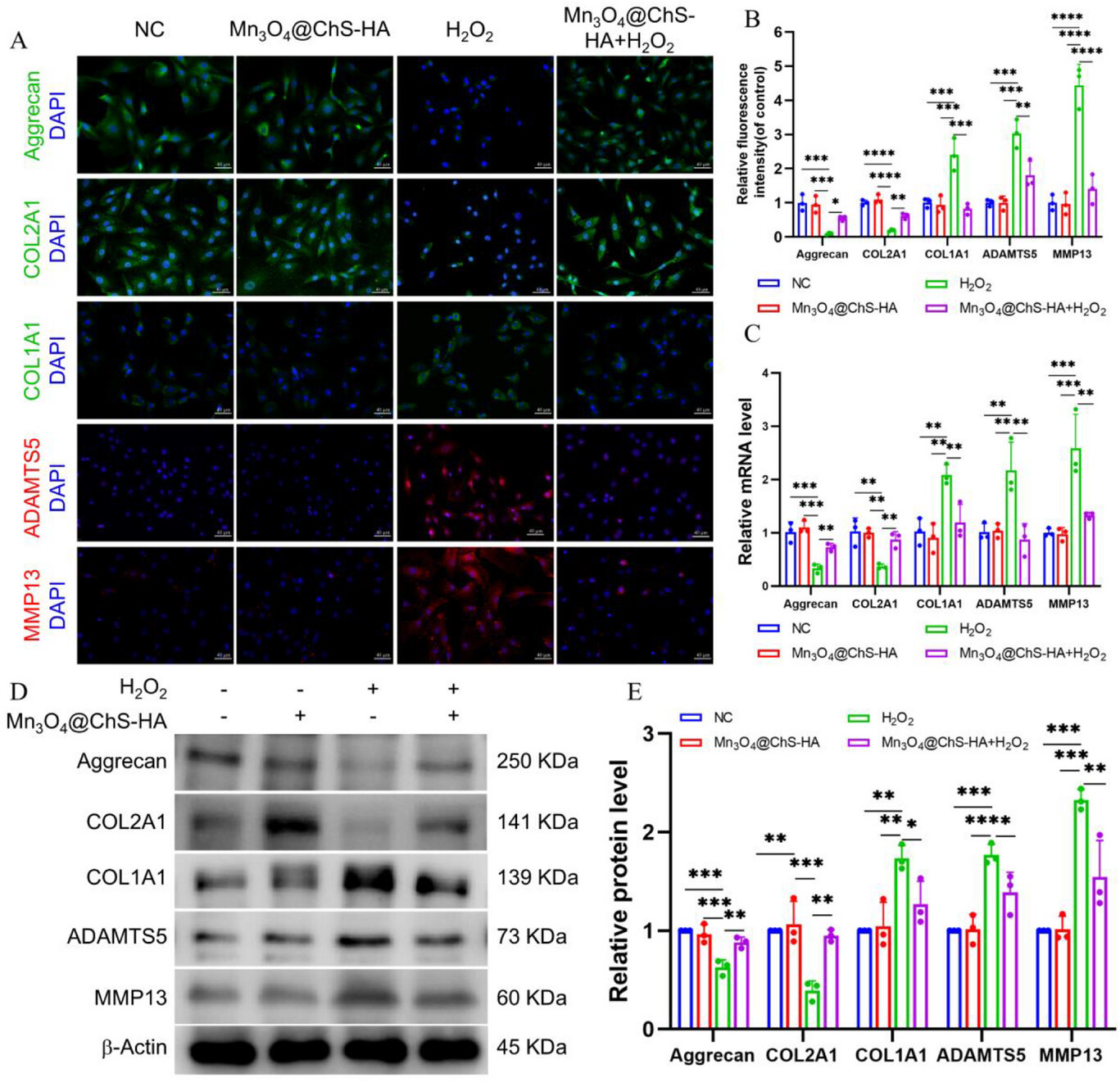
Figure 5. Ability of Mn3O4@ChS-HA to regulate anabolic/catabolic balance.
In the rat model, disc height index (DHI) recovered to 95% of the sham value, paralleling increased proteoglycan deposition. These results suggest that ROS elimination suppresses NF-κB signaling, down-regulating transcription of matrix-degrading enzymes and directly reversing ECM metabolic imbalance. Restoration of ECM components positively correlates with recovery of disc biomechanical function, confirming the necessity of biomaterial implantation for multi-scale tissue reconstruction.
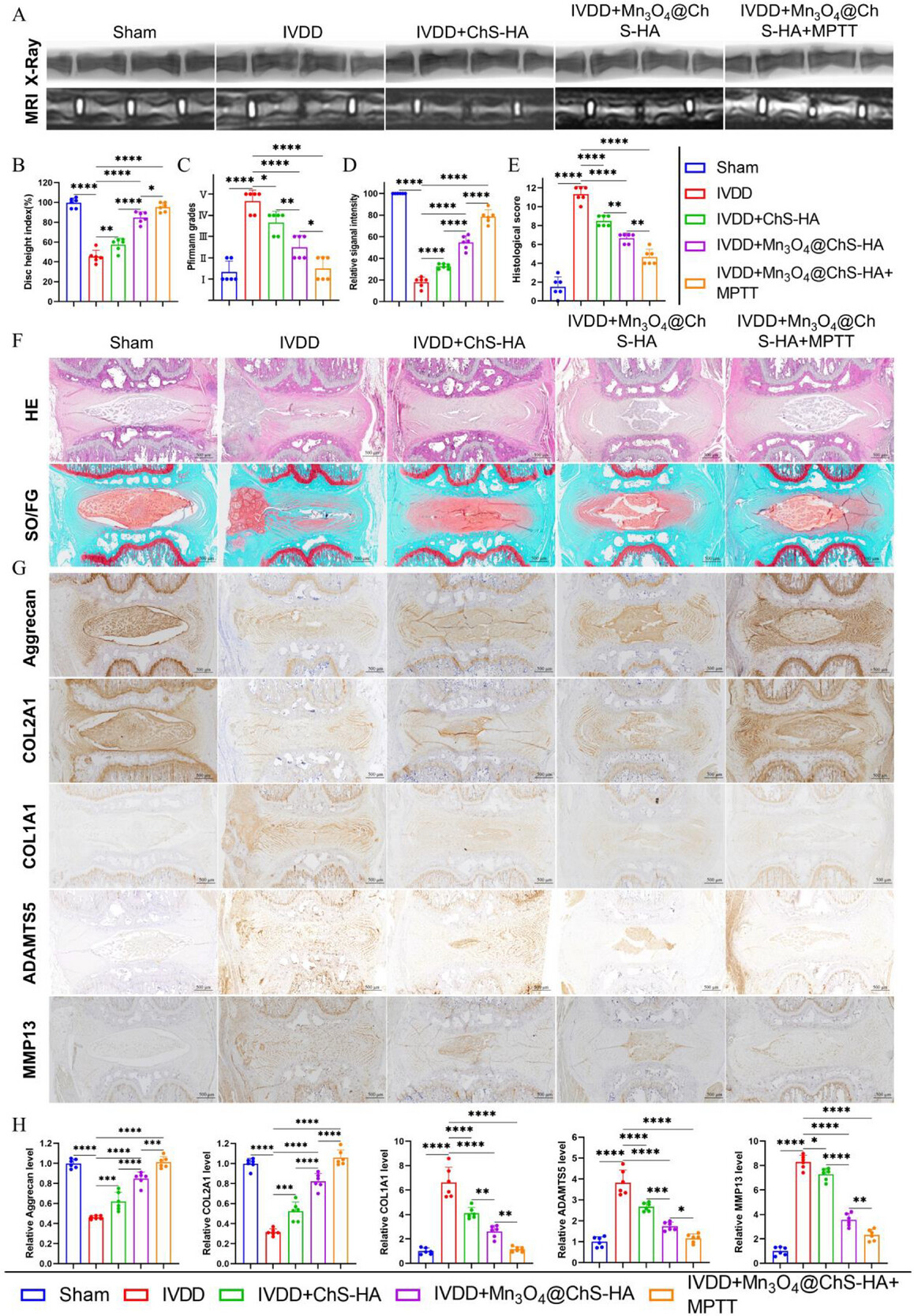
Figure 6. In vivo therapeutic effects of Mn3O4@ChS-HA
KEGG enrichment analysis identified the IL-17 signaling pathway as the most significantly affected gene set. After intervention, IL-17RA protein expression dropped by 65% and correlated negatively with ROS levels. This finding advances two conceptual breakthroughs: (1) it is the first report linking the IL-17 pathway to ECM metabolism in the disc, demonstrating that IL-17 drives matrix degradation via NF-κB–mediated MMP expression; (2) the synergistic antioxidant and mild photothermal anti-inflammatory effects of the nanozyme system more effectively interrupt IL-17 signal transduction.
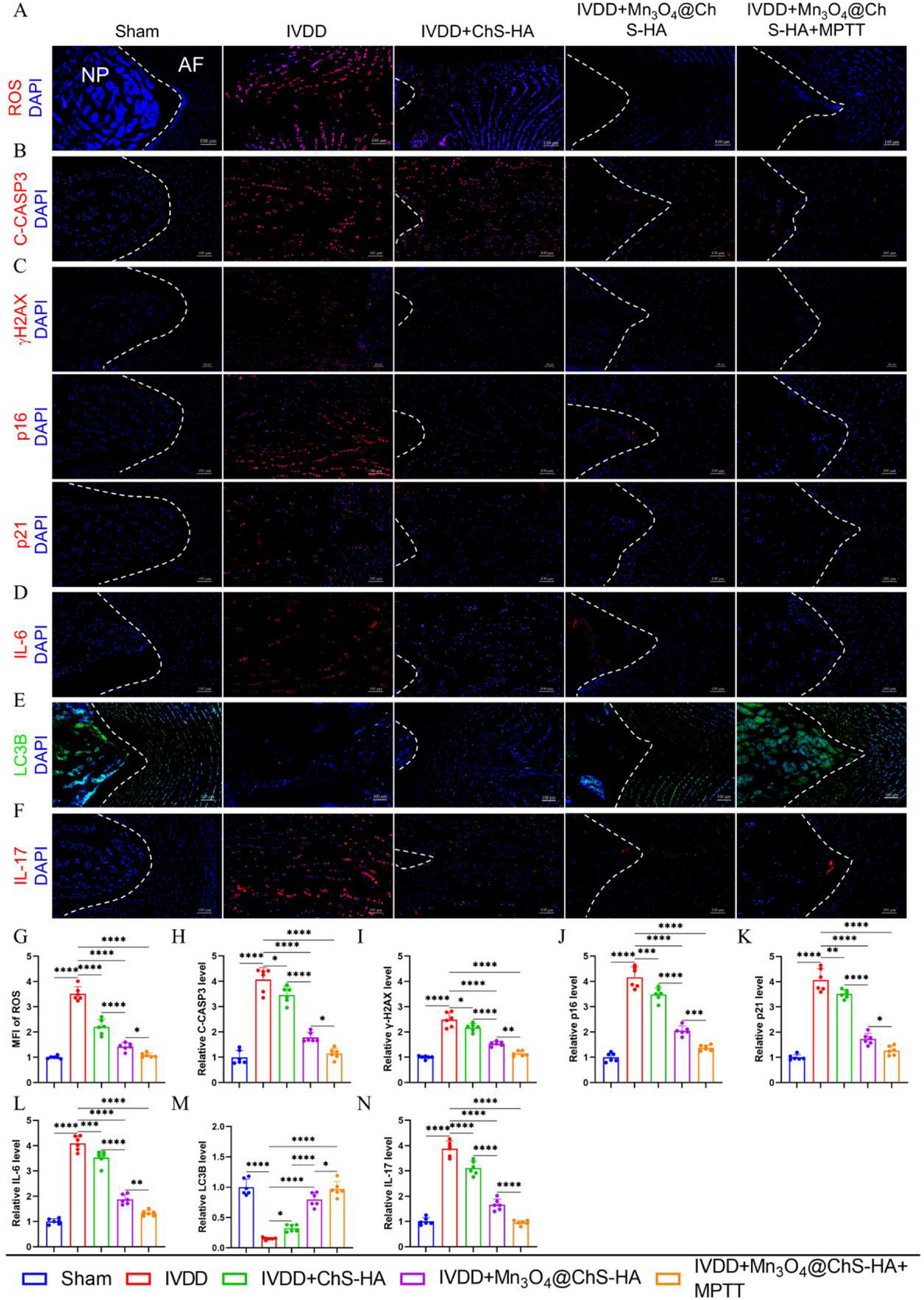
Figure 7. Mn3O4@ChS-HA delays IVDD progression via the IL-17 signaling pathway
This study, through triple-enzyme mimetic design, confirms the necessity of synergistic antioxidant strategies for complex pathological microenvironments, overturning the traditional “single antioxidant enzyme is sufficient” hypothesis. The established “oxidative stress–autophagy dysfunction–ECM degradation” network expands IVDD therapeutic targets from single apoptosis mechanisms to a multi-dimensional regulatory system. Crucially, multi-scale validation quantitatively links material functionality with biological outcomes, providing a new paradigm for evaluating biomaterial efficacy.
Conclusion
The MPTT-nanozyme-hydrogel system (Mn3O4@ChS-HA) leverages redox homeostasis and tissue repair to exert multifaceted biological effects, offering a promising strategy for IVDD treatment. Future work should refine hydrogel design to better replicate the native AF anisotropic architecture and validate efficacy in large-animal models to accelerate clinical translation.
DNA-related Products
|
Catalog |
Product Name |
|
RGK60001 |
Anti-DNA-RNA Hybrid Antibody(S9.6) |
|
RGK60301 |
Anti-DNA G-quadruplex Structures Antibody (BG4) |
|
RGK60307 |
Anti-DNA/RNA G-quadruplex Structures Antibody (BG4) |
|
RGK24302 |
Anti-Z-DNA Antibody (Z22) |
|
RGK24001 |
Anti-DNA Antibody (33.C9) |
|
RGK23951 |
Anti-dsDNA/ssDNA Antibody (3D8) |
|
RGK23953 |
Anti-DNA Antibody (71F12) |
|
RGK24113 |
Anti-DNA Antibody (10F4) |
|
RGK24202 |
Anti-dsDNA/ssDNA Antibody (4B2) |
|
RGK24602 |
Anti-Triplex DNA Antibody (Jel318) |
|
RGK24601 |
Anti-Triplex DNA Antibody (Jel466) |
|
RGK60306 |
Anti-G-quadruplex DNA Antibody (1H6) |
|
RGK24901 |
Anti-dsRNA Antibody (1D3) |
|
RGK25001 |
Anti-ssRNA Antibody (JEL103) |
|
RGK25003 |
Anti-ssRNA Antibody (cAbBC1rib3) |
|
RGK24906 |
Anti-dsRNA Antibody (J2) |
