July 23, 2025 — Diego V. Bohórquez’s team at Duke University published a landmark study in Nature titled "A gut sense for a microbial pattern regulates feeding," revealing for the first time a neural mechanism for rapid microbial sensing in the gut. Termed "neurobiotic sense," this system enables the brain to receive real-time chemical signals from gut microbiota, acting like a "sixth sense" to regulate feeding behavior.

Background
Diego V. Bohórquez has long investigated gut-brain axis neural mechanisms. In 2018 (Science), his team identified specialized neuropod cells in the gut epithelium that directly sense nutrients and transmit signals via the vagus nerve to the brainstem, enabling real-time feeding control. Subsequent work (Nat Neurosci, 2022) elucidated how these cells mediate sugar preference.
Key Findings
1. TLR5 Localization and Function in Colonic Neuropod Cells
Single-cell RNA sequencing revealed that the microbial pattern recognition receptor TLR5 is specifically enriched in PYY-expressing neuropod cells (not immune cells) in the colon, with expression increasing by 57% from ileum to colon. While prior studies showed that global TLR5 knockout in gut epithelial cells causes obesity and inflammation, this study demonstrated that mice lacking TLR5 only in PYY⁺ cells exhibited increased food intake and weight gain without metabolic dysfunction or inflammation. This confirms TLR5 regulates feeding via non-immunological pathways.
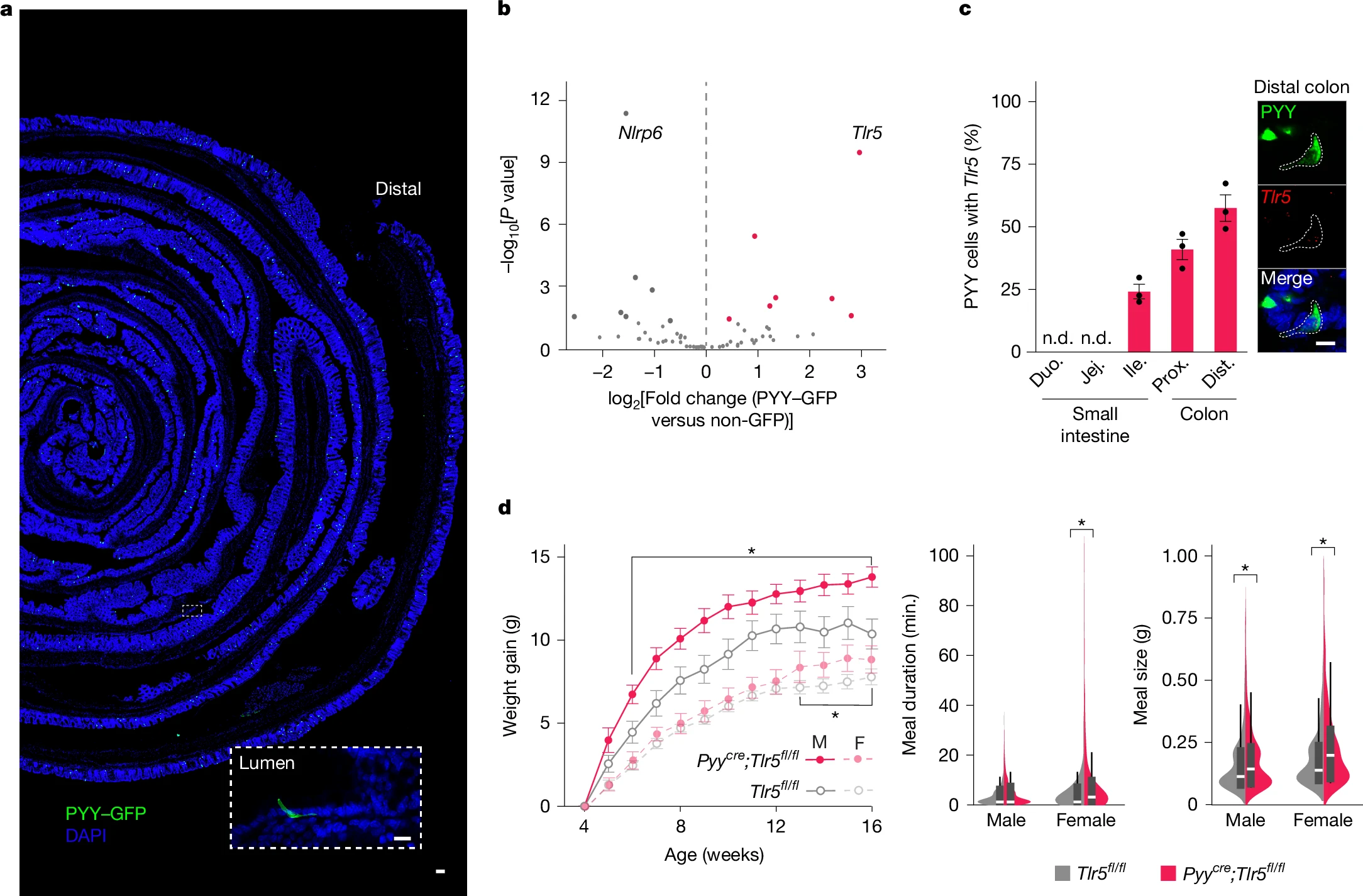
Figure 1. The absence of microbial pattern recognition receptor TLR5 in colonic PYY-labelled cells increases food intake
2. Millisecond-Scale Signal Transduction: Flagellin → PYY → Vagus Nerve
Flagellin activated calcium signaling in PYY⁺ cells and triggered PYY release, a process blocked by TLR5 inhibitors. Optogenetics confirmed direct synapses between PYY⁺ cells and vagal neurons: 473-nm light stimulation of colonic PYY cells induced millisecond-scale vagus nerve activation.
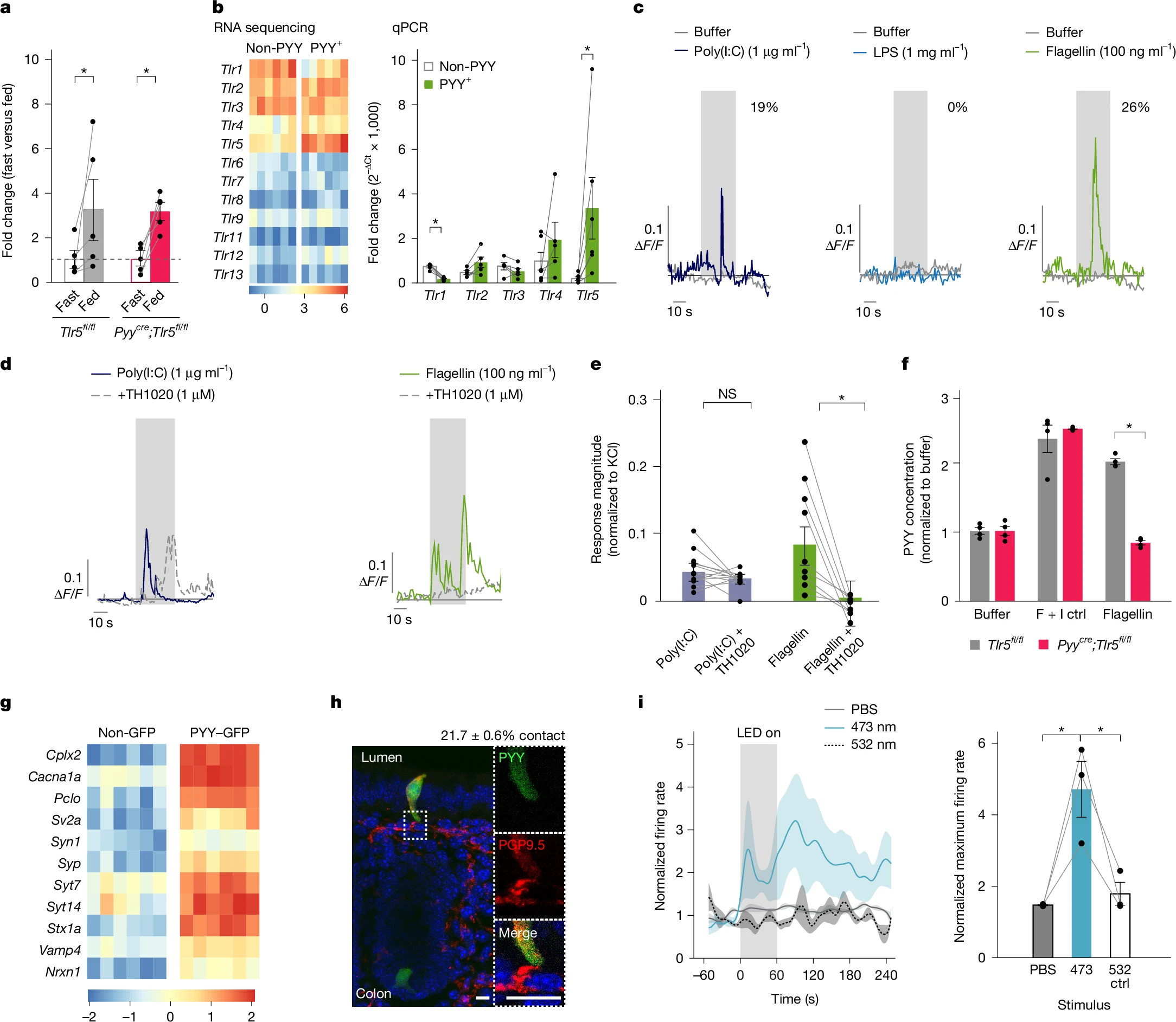
Figure 2. PYY-labelled cells sense microbial flagellin through TLR5
Conversely, silencing PYY⁺ cells or blocking the PYY receptor NPY2R abolished vagal responses to flagellin . In vivo calcium imaging showed 61% of vagal neurons responded exclusively to flagellin, proving a dedicated microbial-sensing pathway.
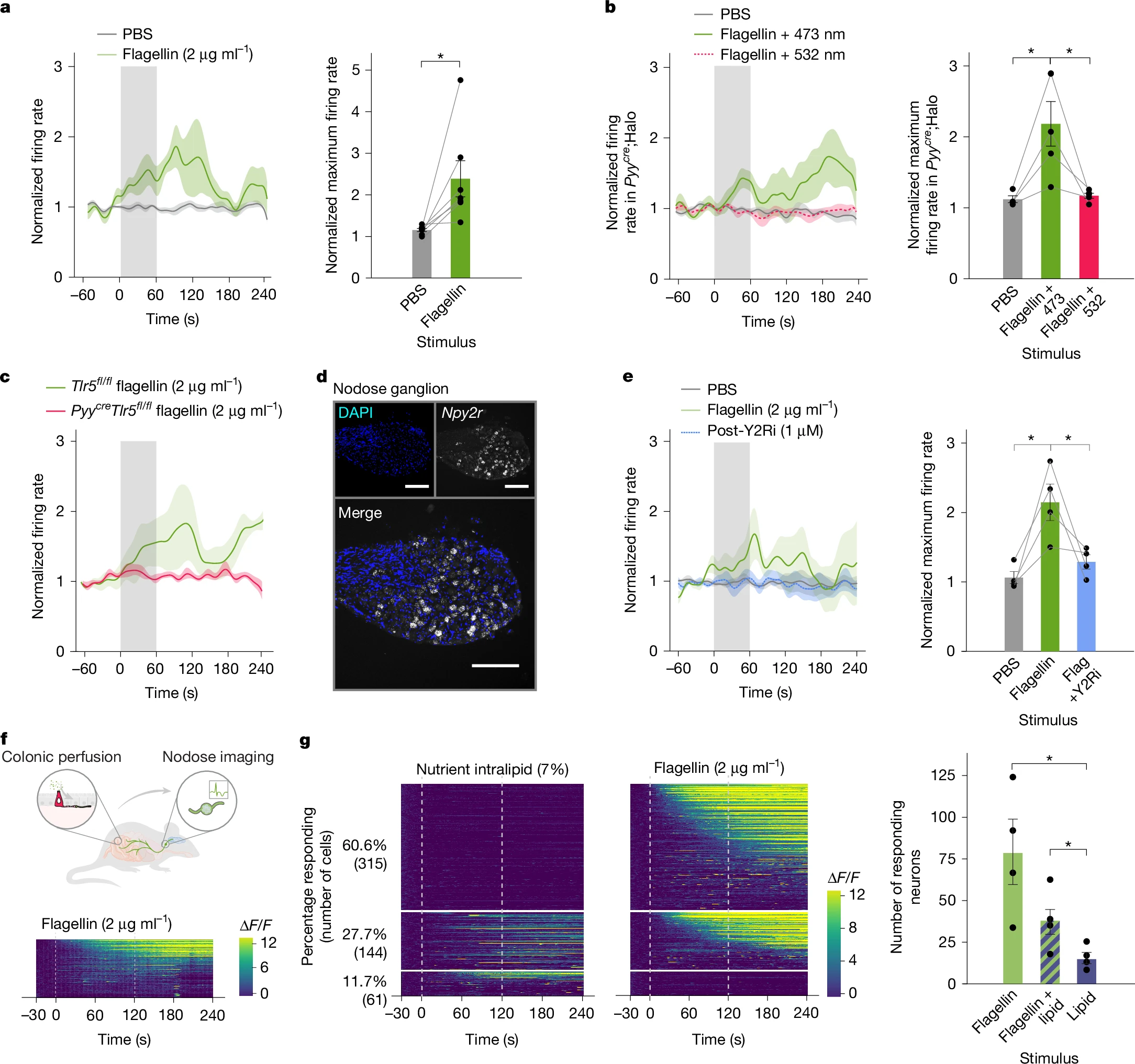
Figure 3. PYY–vagal circuits transduce colonic flagellin
3. Evidence for Direct Microbial Behavior Control: Neurobiotic Sense
Colonic perfusion of flagellin suppressed feeding in wild-type mice within 20 minutes but not in TLR5 knockouts. This effect exhibited three critical properties:
Microbiota-independence: Flagellin inhibited feeding in germ-free mice.
Immune response-independent: No elevated inflammatory cytokines post-perfusion.
Behavioral specificity: The "Crunch Master" audiovisual system revealed flagellin delayed feeding onset without altering bite frequency/duration
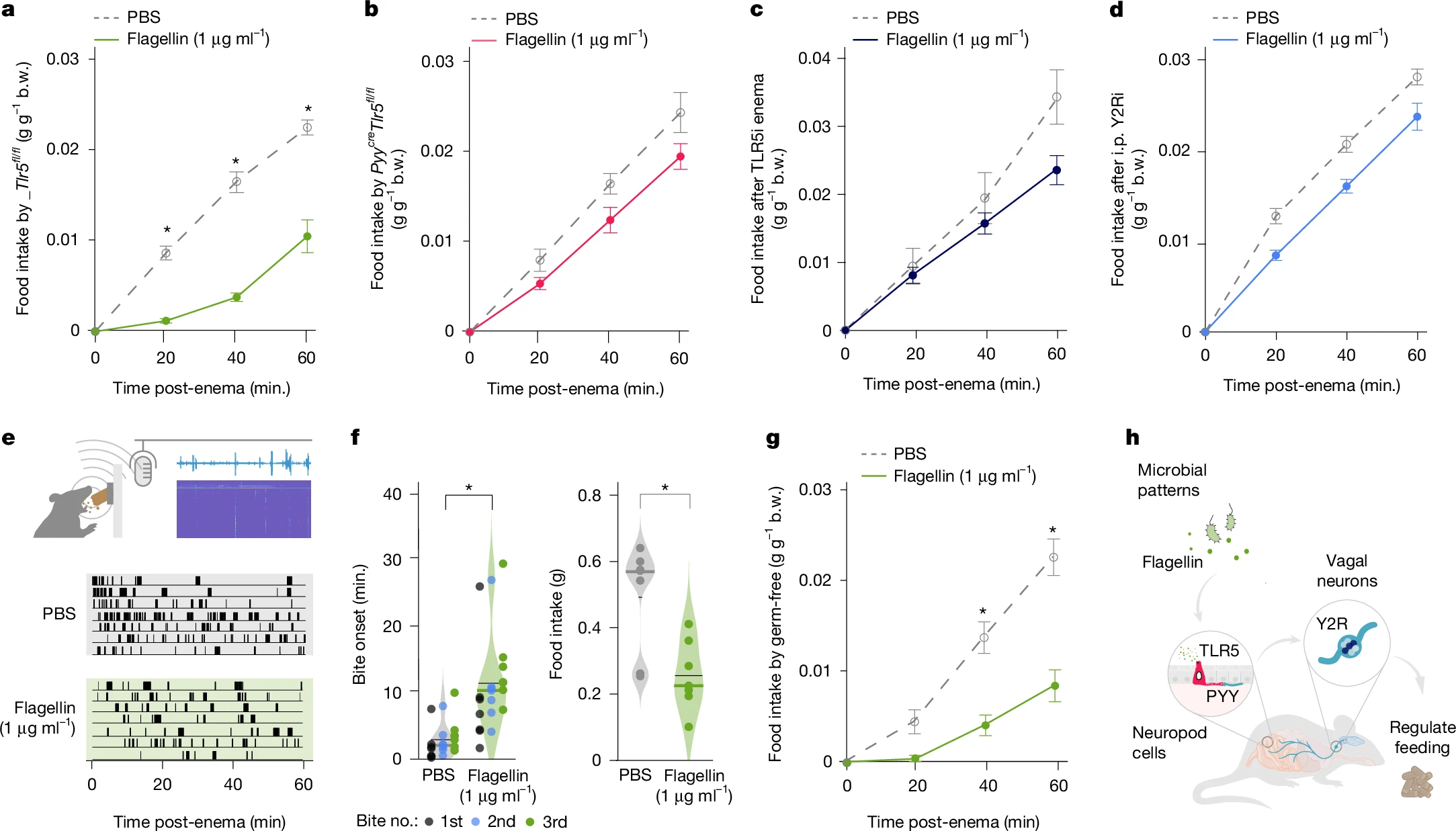
Figure 4. PYY–vagal circuits use TLR5 and Y2R to drive changes in food intake
The study defines a new gut-brain pathway for direct microbial pattern sensing:
Microbial flagellin → TLR5/PYY⁺ neuropod cells in colon → PYY release → NPY2R⁺ vagal neurons → Brainstem → Feeding suppression
This bypasses traditional immune/metabolic intermediaries, enabling real-time behavior regulation by microbial cues and redefining host-microbiota symbiosis.
Bohórquez’s decade-long research has mapped the gut-brain "real-time dialogue" circuitry. By integrating microbes into this framework, the discovery of neurobiotic sense marks a milestone in enteric neuroscience.
Recombinant Protein
|
Catalog |
Product Name |
|
YHA95401 |
Recombinant Human TLR5 Protein, N-His |
|
YHA95402 |
Recombinant Human CD285/TLR5 Protein, N-GST |
Antibody
|
Catalog |
Product Name |
|
PHA95401 |
Anti-Human TLR5 Polyclonal Antibody |
|
PHA95402 |
Anti-Human CD285/TLR5 Polyclonal Antibody |
|
RHA95401 |
Anti-CD285/TLR5 Antibody (R1E71) |
|
RHB93903 |
Anti-Human NPY/PYY Antibody (5E12#) |
|
|
|
|
