Ferroptosis is executed by phospholipid peroxidation, a process relying on metabolic products reactive oxygen species (ROS), phospholipid containing polyunsaturated fatty acid chain(s) (PUFA-PL), and transition metal iron, and that intra- and intercellular signaling events, morphologically, ferroptosis induces rupture of the plasma membrane and cytoplasmic swelling/edema, alongside disruption of mitochondrial morphology, among other alterations, and environmental stresses can impact ferroptosis by regulating cellular metabolism and ROS level.
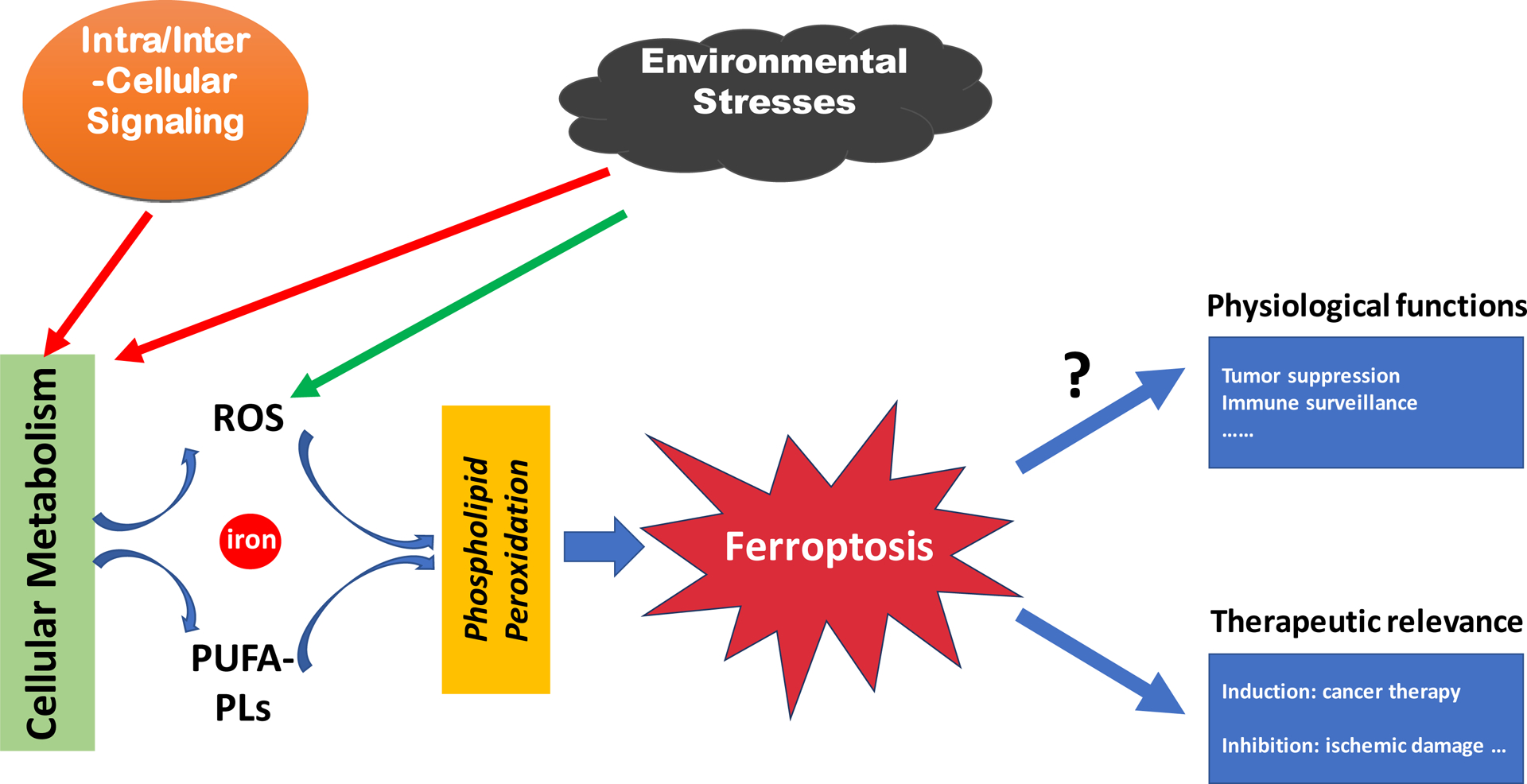
Figure 1. An overview of ferroptosis
(Nat Rev Mol Cell Biol. 2021 Apr;22(4):266-282)
In recent years, rapid progress has been made in understanding the mechanisms of Ferroptosis. Importantly, all these studies have focused on cellular metabolism and have revealed a close relationship between Ferroptosis and metabolic pathways.
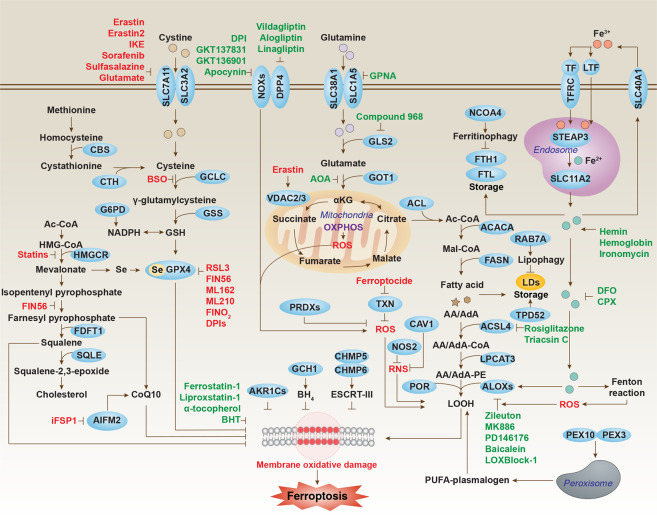
Figure. 2. Core molecular machinery and signaling regulation of ferroptosis
(Cell Res. 2020 Dec 2;31(2):107–125.)
1. Classical Cystine/GSH/GPX4 regulatory pathway
The classical Cystine/GSH/GPX4 regulatory axis is the core pathway governing ferroptosis. This pathway initiates at the plasma membrane system xc⁻ cystine/glutamate antiporter, which transports extracellular cystine into the cell while exporting intracellular glutamate outwards. Intracellular cystine is rapidly reduced to cysteine, the essential precursor for synthesizing the key intracellular antioxidant peptide glutathione (GSH). Synthesized GSH acts as a crucial cofactor, providing reducing equivalents to the pivotal antioxidant enzyme glutathione peroxidase 4 (GPX4). GPX4 utilizes GSH to continuously reduce toxic lipid hydroperoxides (L-OOH) on cellular membranes into harmless lipid alcohols (L-OH), thereby protecting membrane integrity from oxidative damage and preventing ferroptosis. Disruption of any component in this pathway – such as inhibiting system xc⁻ with compounds like Erastin or sulfasalazine (leading to cystine import deficiency and GSH depletion), or directly inhibiting GPX4 activity with agents like RSL3 – results in the loss of cellular capacity to eliminate lipid hydroperoxides. This causes uncontrolled accumulation of lipid peroxides, ultimately triggering the iron-dependent membrane disintegration characteristic of ferroptosis. Consequently, the integrity of this axis represents the primary cellular defense mechanism against ferroptosis.
2. Lipid Metabolism Pathway
The core of the lipid metabolism pathway in ferroptosis lies in the peroxidation of specific polyunsaturated fatty acids (PUFAs) within membrane phospholipids, which is the key execution event of ferroptosis. This pathway involves several critical steps: First, cells need to uptake or synthesize long-chain PUFAs (such as arachidonic acid (AA) or adrenic acid (AdA)); next, acyl-CoA synthetase long-chain family member 4 (ACSL4) activates these free PUFAs into PUFA-CoA esters; then, enzymes like lysophosphatidylcholine acyltransferase 3 (LPCAT3) esterify and incorporate the activated PUFA-CoA into membrane phospholipids (especially phosphatidylethanolamines (PE)), forming PUFA-enriched membrane phospholipids (PUFA-PLs); precisely these PUFA-enriched phospholipids, due to their bis-allylic structures (containing hydrogen atoms easily abstracted by radicals), become primary targets for attack by reactive oxygen species (ROS), particularly hydroxyl radicals generated via the iron-dependent Fenton reaction, making them highly susceptible to lipid peroxidation, generating destructive lipid hydroperoxides (L-OOHs); when the cell's intrinsic antioxidant systems (such as GPX4) fail to timely clear these accumulating lipid hydroperoxides, they disrupt membrane integrity and transmit death signals, ultimately leading to ferroptosis. Therefore, the activity of ACSL4 and LPCAT3, as well as the content of PUFA-PLs in membrane phospholipids, directly determine a cell's sensitivity to ferroptosis.
3. NADPH-Cytochrome P450 Oxidoreductase (POR)-Lipoxygenase (LOXs) Pathway
NADPH provides electrons to LOXs (such as ALOX15, ALOX5) via POR, driving LOXs to catalyze the oxidation of PUFAs (like arachidonic acid), generating lipid hydroperoxides. This process is independent of GPX4 and promotes ferroptosis by directly driving lipid peroxidation. For example, ALOX15 can be induced in various cell types, and its overactivation accelerates the lipid peroxidation chain reaction, serving as one of the key drivers of ferroptosis.
4. Iron Metabolism Pathway
The core of the iron metabolism pathway lies in the aberrant accumulation of intracellular reactive divalent iron ions (Fe²⁺), which is the key catalyst driving the lipid peroxidation chain reaction. This pathway involves imbalances in iron uptake, storage, and utilization: increased iron uptake mediated by the transferrin receptor (TFRC), or decreased iron storage capacity mediated by ferritin (e.g., through autophagic degradation of ferritin releasing iron), lead to elevated levels of free Fe²⁺ in the intracellular labile iron pool (LIP); excess Fe²⁺ catalyzes the decomposition of lipid hydroperoxides (L-OOHs) via the Fenton reaction, generating highly reactive lipid radicals (LO• and LOO•); these radicals further attack PUFAs within membrane phospholipids, initiating and amplifying the chain reaction of lipid peroxidation, ultimately resulting in oxidative disintegration of the cell membrane and ferroptosis; therefore, iron ions are not only a hallmark but also an indispensable catalyst in the execution of ferroptosis.
5. Mitochondria-Related Pathways
During ferroptosis, mitochondria often exhibit characteristics such as reduced volume, reduced or absent cristae, and outer membrane rupture. Abnormal activity of mitochondrial respiratory chain complexes I and III can promote reactive oxygen species (ROS) generation, exacerbating lipid peroxidation; simultaneously, imbalances in mitochondrial membrane potential and disruption of the tricarboxylic acid (TCA) cycle may indirectly promote ferroptosis by affecting energy metabolism and redox status.
Beyond the pathways described above, ferroptosis is also regulated by factors such as cellular metabolism, the AMPK signaling pathway, autophagy, and hypoxia signaling transduction. Although the physiological functions of ferroptosis remain unclear, current research demonstrates its critical regulatory role in various diseases. Besides cancer and ischemic organ injury, ferroptosis is also implicated in the pathogenesis of an increasing number of other diseases, such as neurodegeneration, hepatic and pulmonary fibrosis, autoimmune diseases, and Mycobacterium tuberculosis-induced tissue neurodegeneration, hepatic and pulmonary fibrosis, autoimmune diseases, and Mycobacterium tuberculosis-induced tissue necrosis and so on.
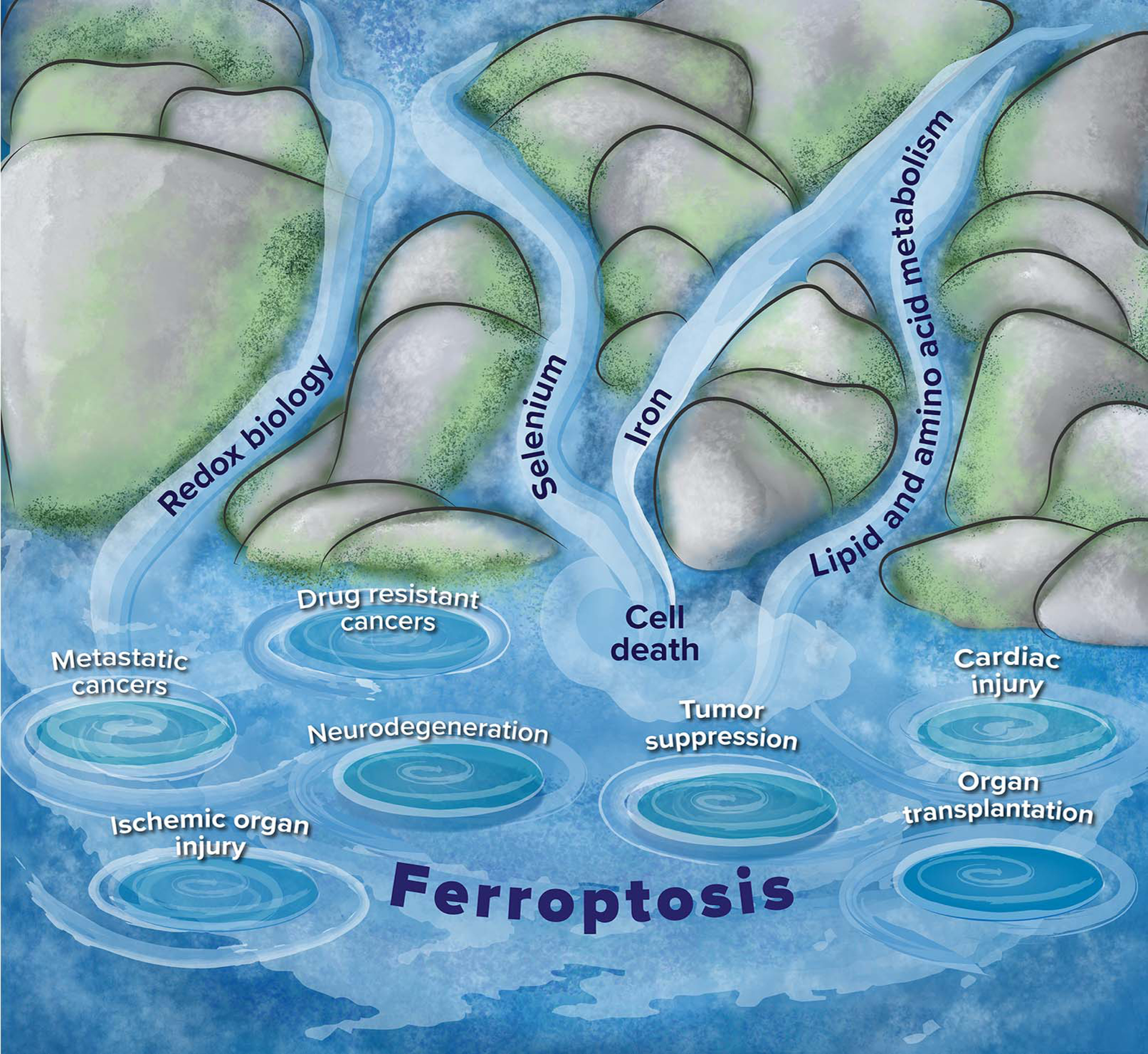
Figure 3. Relationship of ferroptosis to other biological processes and diseases
(Nat Rev Mol Cell Biol. 2021 Jan 25;22(4):266–282)
References
1. Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nature Reviews Molecular Cell Biology. 2021;22.
2. Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Research.
3. Liu J, Kang R, Tang D. Signaling pathways and defense mechanisms of ferroptosis. The FEBS Journal. Published online June 17, 2021.
Product List:
Recombinant Protein
|
Catalog |
Product Name |
|
Recombinant Human SLC7A11/xCT Protein, N-His |
|
|
Recombinant Human GPX4 Protein, N-GST |
|
|
Recombinant Human GPX4/PHGPx Protein, N-GST |
|
|
Recombinant Human FTH1 Protein, N-His |
|
|
Recombinant Human ACSL4 Protein, N-His |
|
|
Recombinant Human CD71/TFRC Protein, C-His |
|
|
Recombinant Human NFE2L2 Protein, N-His |
Antibody
|
Catalog |
Product Name |
|
Anti-NFE2L2 Antibody (R3U23) |
|
|
Anti-NFE2L2 Polyclonal Antibody |
|
|
Anti-SLC7A11/xCT Antibody (R2L62) |
|
|
Anti-SLC7A11/xCT Antibody (R2L63) |
|
|
Anti-GPX4 Polyclonal Antibody |
|
|
Anti-Human GPX4/PHGPx Antibody (SAA0487) |
|
|
Anti-GPX4/PHGPx Antibody (R1V41) |
|
|
Anti-GPX4/PHGPx Antibody (R1V42) |
|
|
Anti-FTH1 Antibody (R1J25) |
|
|
Anti-FTH1 Antibody (R1J26) |
|
|
Anti-Human Serum Ferritin/SF Monoclonal Antibody (1A281) |
|
|
Anti-ACSL4 Antibody (R1E61) |
|
|
Anti-ACSL4 Antibody (R1E62) |
|
|
Anti-ACSL4 Polyclonal Antibody |
|
|
Anti-Mouse CD71/TFRC Antibody (R17217) |
|
|
Anti-Phospho-NFE2L2/Nrf2 (Ser40) Antibody (R2G84) |
More research tools related to ferroptosis can be found here
