Three oncologists at Hong Kong's Princess Margaret Hospital earlier developed acute gastroenteritis, after which one died. The Hong Kong Center for Health Protection released the results of its investigation last week and found that there was no link between the three people's illnesses. Yuen Kwok-yung, Chair Professor of Infectious Diseases at the Department of Microbiology of the University of Hong Kong, who was in charge of the investigation, said that the team found rotavirus type C in different samples from the deceased doctors, and concluded that the signs of the disease that appeared in the patients before their deaths were caused by the virus.
Rotavirus (RVs) are non-enveloped double-stranded RNA (dsRNA) viruses that belong to the Reoviridae family. Their genome comprises 11 segments of dsRNA encoding six viral structural proteins (VP1 to VP4, VP6 and VP7) and six non-structural proteins (NSP1 to NSP6).
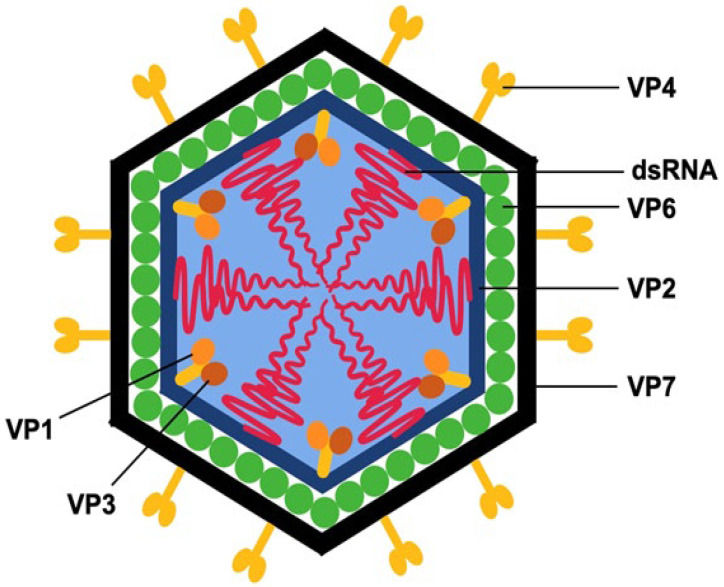
Localization of RV proteins within the viral particle (Pathogens. 2025 May 14;14(5):480)
Structural (VP) and non-structural (NSP) RV proteins play essential roles in viral replication, the manifestation of clinical symptomatology, and the classification of RV species.
Table 1. Functional characterization of RV structural and non-structural protein
|
Name of Protein |
Role of the Protein |
|
Structural proteins (VP) |
|
|
VP4, VP7 |
Mediate viral attachment to host cell surface receptors and facilitate membrane penetration during entry. VP4 contributes to viral antigenic diversity, with 58 recognized P genotypes . |
|
VP1 |
Functions as the RNA-dependent RNA polymerase, catalyzing the synthesis of viral RNA. |
|
VP2 |
Serves as the inner capsid scaffold and essential cofactor for VP1, enabling initiation of double-stranded RNA genome replication. |
|
VP3 |
Guanylyl-methyltransferase, capping enzyme. RV proteins NSP1 and VP3 act to suppress interferon (IFN) expression by inducing degradation of transcription factors and other host elements essential for effective innate immune responses. |
|
VP6 |
Major structural protein. Involved in RV diversity—42 G-types and species classification (RVA–RVJ). |
|
Non-structural proteins (NSP) |
|
|
NSP1 |
Interferon antagonist. |
|
NSP2 |
Involved in RV particle assembly. |
|
NSP3 |
Stimulates translation of both capped and uncapped viral mRNA. |
|
NSP4 |
Involved in RV particle assembly, enterotoxin. |
|
NSP5 |
Involved in RV particle assembly. |
|
NSP6 |
Expressed from an alternative reading frame of the NSP5 gene in some Group A RV strains. |
Rotavirus type A is widely prevalent in the population, with relatively low levels of prevalence for RV types B, C and H. RV type C was first identified in pigs and subsequently in humans. Animal C-RV is an important pathogen in swine gastroenteritis, while C-RV can also infect dogs, cattle, and ferrets. Human C-RVs are the second most common species of RVs in humans, and unlike A-RVs, which primarily infect infants and young children, C-RVs can also infect children.
The primary site of RV A infection is the gastrointestinal tract, with the virus being shed in the feces of infected individuals for a period of 5 to 7 days. Transmission primarily occurs via the fecal-oral route. Direct contact—particularly hand-to-hand—may also facilitate spread. Although the risk of zoonotic transmission of RV from animals to humans is low, such transmissions are unlikely to cause significant clinical illness. RVs exhibit substantial resistance to environmental stressors, including temperature and pH, which enhances their infectious potential. RV infection primarily manifests as AGE, characterized by diarrhea and vomiting, which can lead to systemic complications such as dehydration. Additionally, recent studies indicate the potential role of RV in central nervous system complications, autoimmune disorders, biliary atresia, and respiratory diseases.
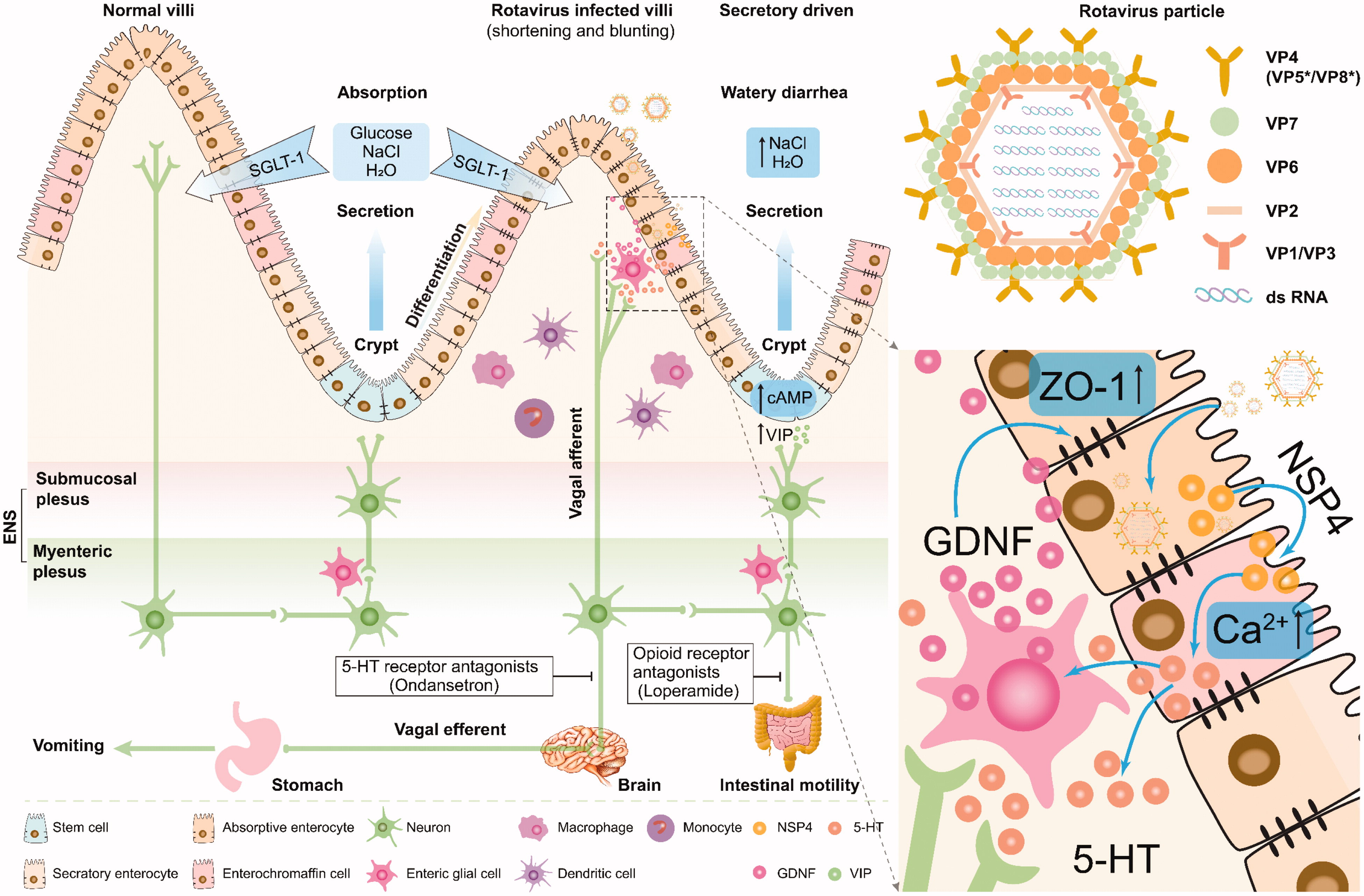
Figure 3. Rotavirus-related systemic diseases and proposed mechanisms(Crit Rev Microbiol. 2021 Sep;47(5):580-595)
Although the introduction of anti-rotavirus vaccines (Rotateq and Rotarix, two live-attenuated oral vaccines) has raised hopes that the virus can eventually be constrained, clinical management of rotavirus-associated disease is complicated by the absence of specific antiviral therapy. In addition, recent studies have demonstrated the potential role of RV in central nervous system complications, autoimmune diseases, biliary atresia, and respiratory disorders.
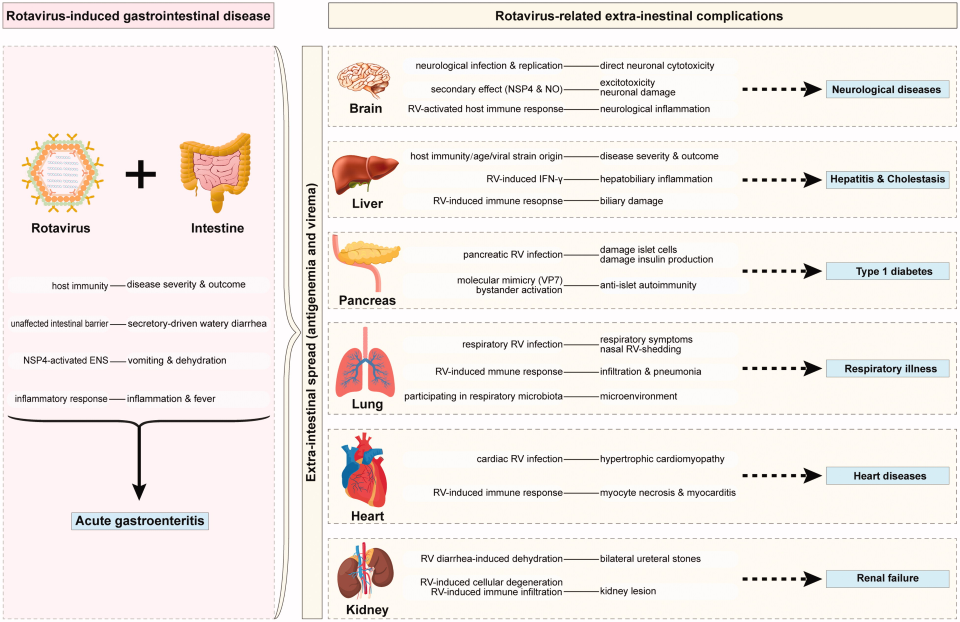
Figure 3. Rotavirus-related systemic diseases and proposed mechanisms(Crit Rev Microbiol. 2021 Sep;47(5):580-595)
RV related Products List:
Recombinant Protein
|
Catalog No. |
Puduct Name |
|
Recombinant Human rotavirus A VP4/Outer capsid protein VP4 Protein, N-His |
|
|
Recombinant PoRV VP6 Protein, N-His |
|
|
Recombinant RV-A VP6 Protein, N-His |
Polyclonal Antibody
|
Catalog No. |
Puduct Name |
|
Anti-Rotavirus A/RV-A VP4 Polyclonal Antibody |
|
|
Anti-Rotavirus A/RV-A VP6 Polyclonal Antibody |
Monoclonal Antibody
|
Catalog No. |
Puduct Name |
|
Anti-Rotavirus/RV Monoclonal Antibody (1A248) |
|
|
Anti-Rotavirus/RV Monoclonal Antibody (1A249) |
|
|
Anti-Rotavirus/RV Monoclonal Antibody (1A250) |
|
|
Anti-Rotavirus/RV Monoclonal Antibody (1A251) |
|
|
InVivoMAb Anti-Rotavirus A VP4/Outer capsid protein VP4 Antibody (Iv0200) |
|
|
InVivoMAb Anti-Rotavirus A/RV-A Outer capsid glycoprotein VP7 (Iv0011) |
|
|
InVivoMAb Anti-Rotavirus A/RV-A Intermediate capsid protein VP6 (Iv0012) |
|
|
InVivoMAb Anti-Rotavirus A/RV-A Outer capsid protein VP4 (Iv0013) |
