Antibodies are powerful tools for protein and molecular detection and purification. While intact antibodies (typically IgG or IgM) are suitable for most immunoassay applications, certain procedures benefit from the use of antibody fragments such as Fab, scFv, and dAb.
Antibody fragments are smaller than full-length antibodies, enabling better tissue penetration for therapeutic or immunohistochemical applications. Most fragments are non-glycosylated and can be produced in prokaryotic expression systems, reducing costs and production time. With the advancement of "precision" antibody drug development, antibody fragments—characterized by their small size, strong penetration, and ease of engineering—have gained prominence in targeted cancer therapy and immunotherapy.
This article explores the types of antibody fragments and their purification methods.
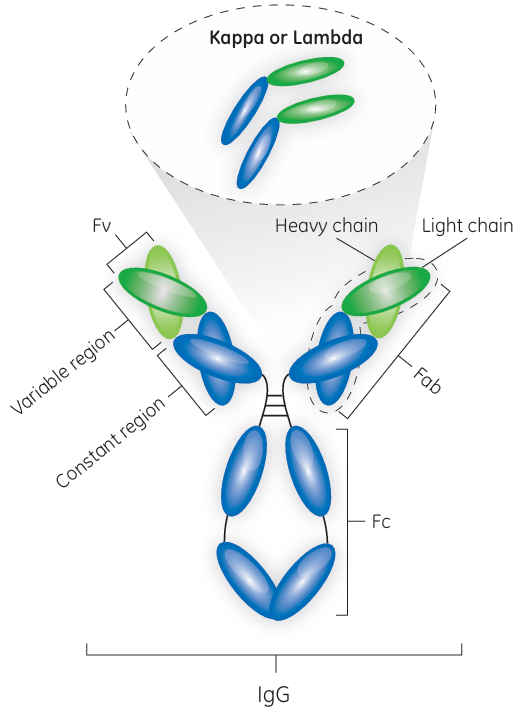
Fig 1. Antibody with marked domain names
Antibody Fragment Types
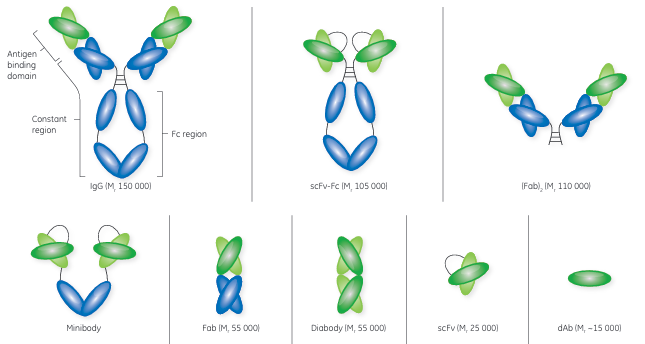
Fig 2. Structure of antibody and antibody fragments.
Fab = fragment, antigen-binding; scFv = single-chain fragment, variable; and dAb = domain antibody
Fabs are considered the first generation of antibody fragments and were initially generated by cleavage of an intact antibody using an enzyme, such as papain. Papain cleavage yields two monovalent Fab fragments, each composed of one variable heavy chain (VH ) and one variable light chain (VL ) linked by disulfide bonds and displaying a single antigen-binding site. Today, Fabs are produced using modern genetic engineering approaches.
The scFvs are monovalent structures, with affinity for a single antigen. With an approximate size of Mr 25 000, an scFv contains the variable regions of an antibody’s heavy and light chains fused into a single polypeptide chain via a short flexible linker. An scFv comprises the complete antigenbinding site of its parental antibody molecule.
Consisting of the VH or VL domains, dAbs are some of the smallest functional antibody fragments that retain full antigen-binding specificity. The dAb is approximately one-tenth of the molecular weight of a normal antibody. Although dAbs contain only three of the six complementary determining regions from the parent antibody, they do exhibit antigen binding specificity and affinity. A dAb can be remarkably stable under harsh conditions of temperature, pressure, and denaturing chemicals.
Antibody fragment expression and purification
Although higher eukaryotic cells have successfully been used to express antibody fragments, their small size and nonglycosylated nature allow the use of simpler and less costly prokaryotic or yeast expression systems. However, microbial expression systems place significant demands on culture clarification and the primary capture step.
Crossflow filtration (CFF) is a suitable technique for clarification of viscous or high-solid feeds such as microbial fermentation broth. Hollow fiber filter cartridges are commonly used for the CFF step. Because of their open channel structure, hollow fiber filters are well-suited for microfiltration applications such as recovery of proteins expressed in bacteria or yeast. Several antibody fragments have been purified using cation exchange chromatography (CIEX) as the capture step.
Capture of antibody fragments containing κ light chain
Protein L is present at the surface of about 10% of Finegoldia magna strains. Attributed to its rather unique binding specificities, Protein L offers options to purification of antibody fragments. Native Protein L is a Mr 76 000 to 106 000 protein, containing four or five highly homologous, consecutive extracellular domains responsible for the protein’s interaction with Ig kappa light chains. Given that its target is the kappa light chain, Protein L will bind to representatives of most antibody classes, including IgG, IgM, IgA, IgE, and IgD. It should be noted, however, that the native form of Protein L does not recognize antibodies (or related fragments) from certain animal species. As the binding site for Protein L is located in the framework region 1 (a less variable region than the CDRs of the variable domain) of the kappa light chain, fragments derived from antibodies that have the kappa light chain can be purified using Protein L. As Protein L interacts with the kappa light chain, it has no immunoglobulin class restrictions. Hence, Protein L offers the potential of being a broadly useful, if not fully as general as Protein A, affinity ligand. Approximately 60% of mammalian IgG light chains are kappa chains, with the remaining 40% being lambda chains that lack binding sites for Protein L.
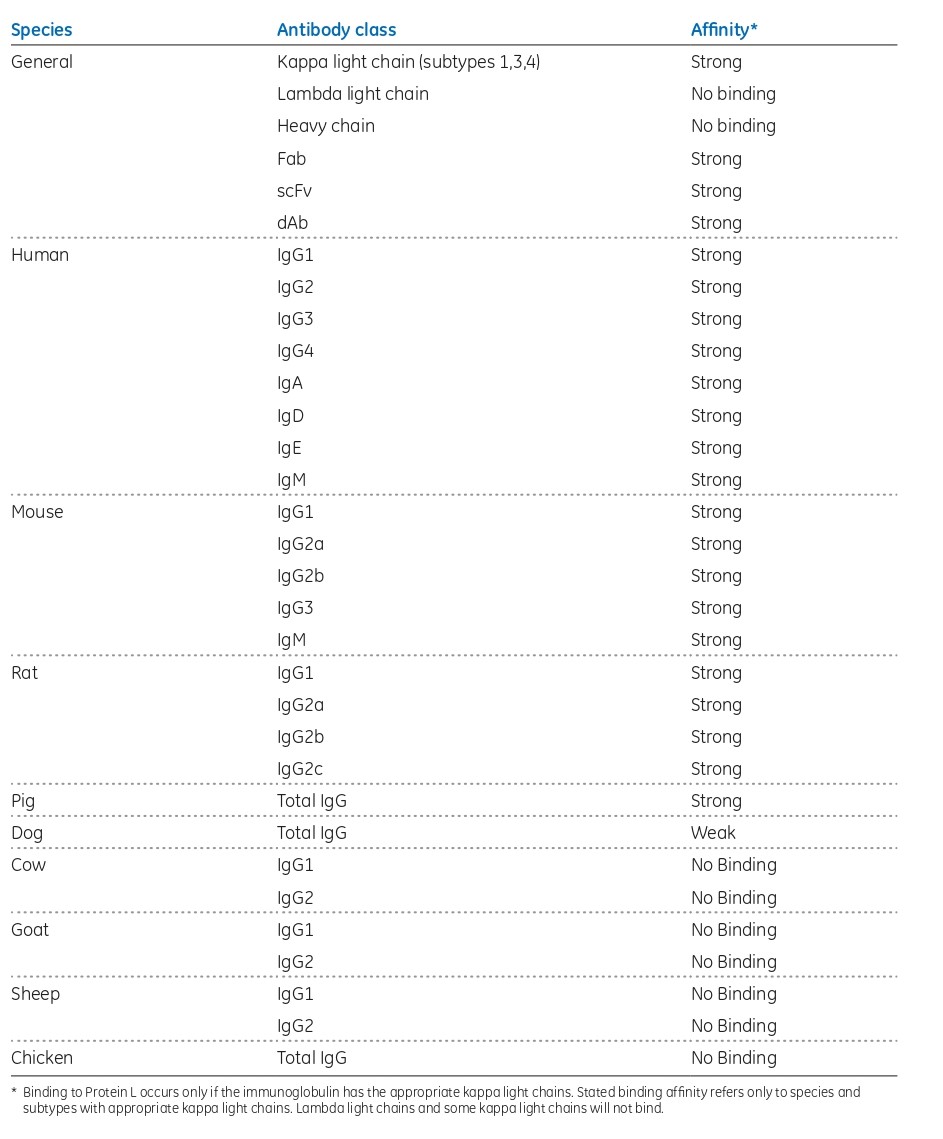
Fig 3. Protein L binding affinities
Protein L-based affinity chromatography resins for research applications have been commercially available for many years. With the introduction of the Capto™ L resin, however,
the first opportunity for an industrial platform for the purification of antibody fragments emerged. With its recombinant Protein L ligand, Capto L is a BioProcess chromatography resin with a broad affinity for a range of antibody fragments of different sizes that contain kappa light chains. With its rigid base matrix, allowing for high flow rates and high productivity, as well as low ligand leakage, Capto L resin is well suited for large-scale manufacturing. The recommended cleaning-in-place (CIP) protocol for Capto L resin is the use of 15 mM NaOH for 15 min, allowing for approximately 100 cleaning cycles with > 90% remaining binding capacity. Due to the high-affinity binding of Protein L to the variable region of the kappa light chain, Capto L purifies conventional Fabs, scFv, and dAbs.
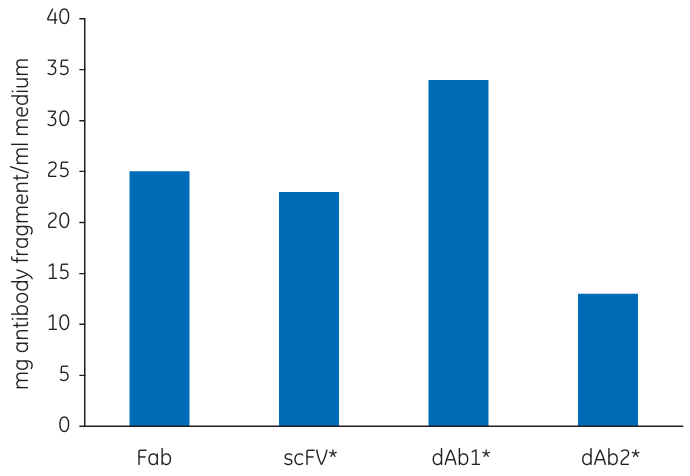
Fig 4. QB10 of Capto L for four human antibody fragments. Fab fragment kindly provided by UCB Celltech
As the DBC is normally measured in mg/mL, the molecular weight of the target molecule is an important factor to consider.
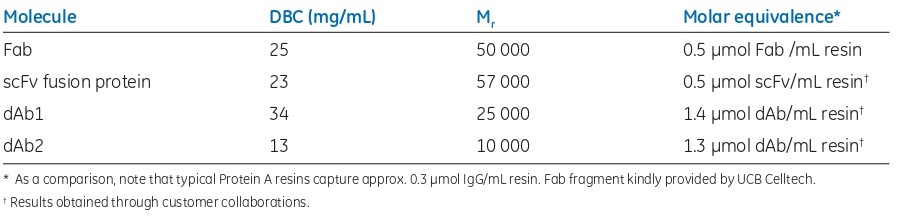
Fig 5. DBC of Capto L for four different human antibody fragments
KappaSelect is an affinity resin that binds to the constant region on the kappa light chain and can be used to capture Fabs containing the kappa light chain under conditions where Capto L is found to be less suitable.
Capture of antibody fragments containing λ light chains
LambdaFabSelect is an affinity resin used for the capture of Fabs containing the lambda light chain. LambdaFabSelect binds to the constant region of the lambda light chain and can therefore bind Fab fragments. Together, Capto L and LambdaFabSelect cover nearly all Fabs as well as a majority of the smaller antibody fragments.
The ligands of the LambdaFabSelect and KappaSelect resins are both based on single-chain antibody fragments with affinity for either human IgG lambda or kappa light chain.
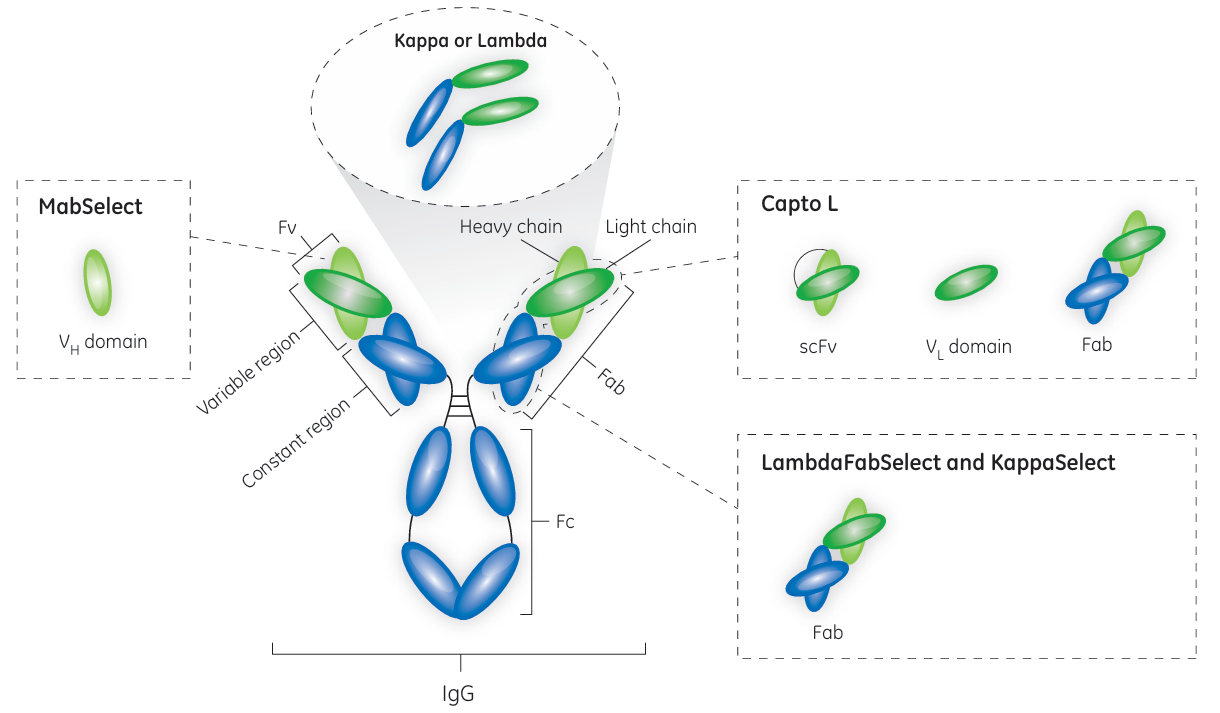
Fig 6. Affinity map for resins included in GE Healthcare’s toolbox for the capture of antibody fragments
Capture of antibody fragments containing heavy chains
In addition to its binding in the Fc region, the recombinant Protein A ligand used in MabSelect™ affinity resin binds to the VH3 domain subtype of human IgG Fabs. Hence, MabSelect resin can be a useful alternative for capturing heavy chain dAbs that contain the VH3 domain subtype.
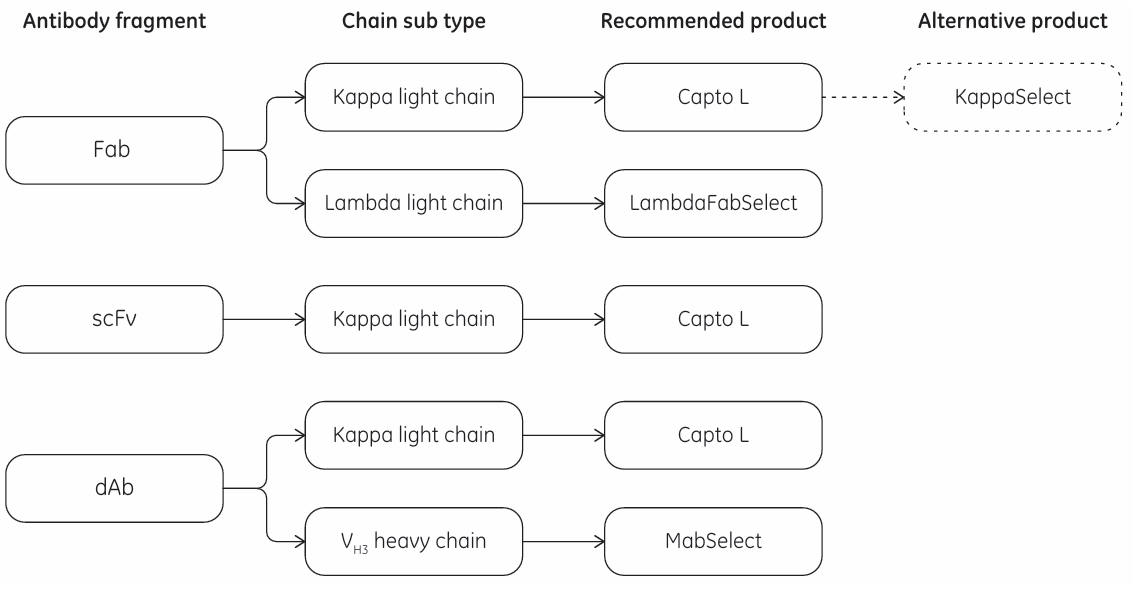
Fig 7. Selection guide of the capture toolkit
