Title: Spermidine mediates acetylhypusination of RIPK1 to suppress diabetes onset and progression
Nat Cell Biol. 2024 Dec;26(12):2099-2114. doi: 10.1038/s41556-024-01540-6. Epub 2024 Nov 7.
Research Background
Type 2 diabetes (T2D) has emerged as one of the fastest-growing global public health challenges, with over 537 million adult patients worldwide—a threefold increase in the past two decades. Characterized by insulin resistance, current clinical interventions focus primarily on glycemic control, yet a lack of systematic understanding of the molecular mechanisms underlying disease progression has hindered the development of breakthrough therapies.
Previous studies identified associations between N-acetyltransferases (murine NAT1 [mNAT1] and human NAT2 [hNAT2]) and insulin sensitivity, but their mechanistic roles remained unclear. NAT1 deficiency reduces intracellular spermidine levels. Spermidine, a natural polyamine linked to longevity and age-related disease prevention, has an undefined role in insulin resistance and diabetes progression.
A study published in Nature Cell Biology titled "Spermidine mediates acetylhypusination of RIPK1 to suppress diabetes onset and progression", reveals a novel post-translational modification—acetylhypusination, on receptor-interacting serine/threonine-protein kinase 1 (RIPK1), a key regulator of inflammation and cell death. This modification inhibits RIPK1 activation, thereby reducing cell death and inflammation. Remarkably, supplementing NAT1-deficient mice with spermidine alleviated RIPK1-mediated cell death and diabetic phenotypes.

This study highlights the role of vascular pathology in T2D pathogenesis and identifies RIPK1 kinase inhibition as a potential therapeutic strategy.
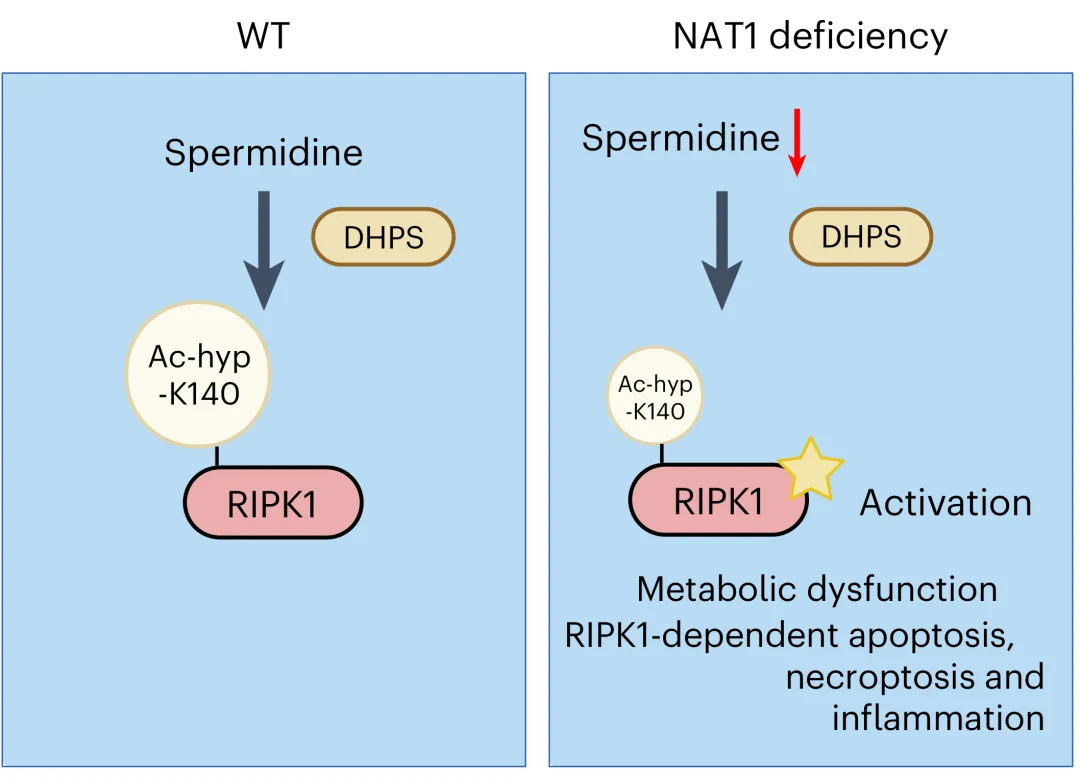
Figure 1. A model for regulating the activation of RIPK1 kinase activation by spermidine-mediated hypusination.
Clinical Relevance
Analysis of vascular tissues from coronary artery bypass patients revealed reduced spermidine levels in T2D patients and activated RIPK1 in diabetic nephropathy biopsies, validating murine findings and confirming spermidine and RIPK1’s roles in human T2D.
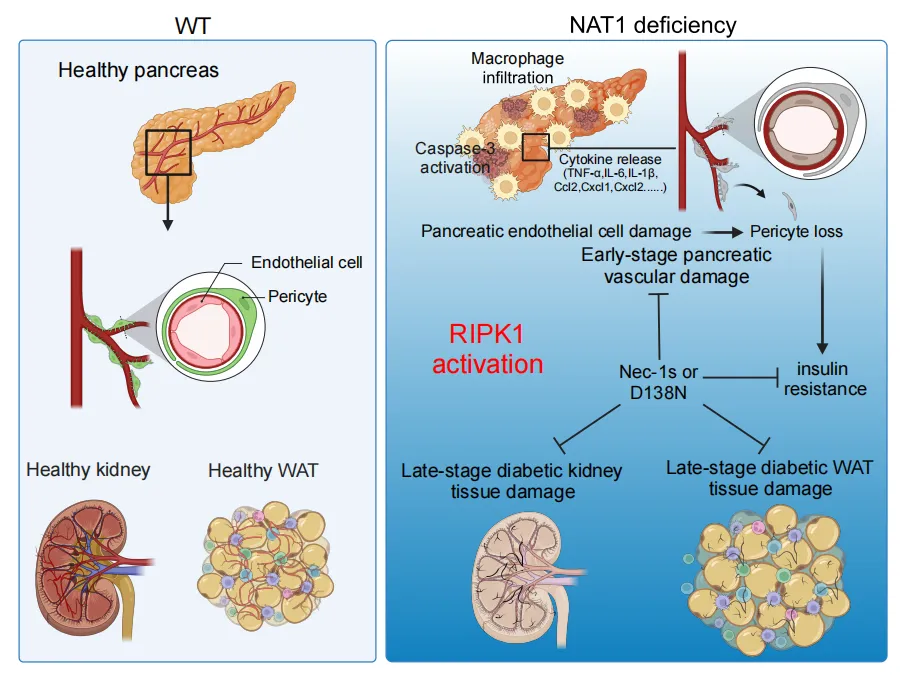
Figure 2. NAT1 deficiency in endothelial cells promotes RIPK1 activation, RDA and necroptosis, which in turn induces vascular damage and inflammation in the pancreas and systemic insulin resistance.
Key Antibody
This study utilized Anti-Hypusine antibody (Hpu24) (Catalog: RGK08101) from AntibodySystem for Western blot analysis.
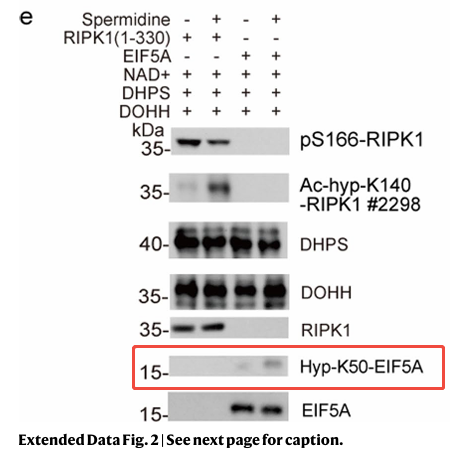
The team employed deoxyhypusine synthase (DHPS) and deoxyhypusine hydroxylase (DOHH) in in vitro hypusination assays. An alkyne-spermidine probe, coupled with click chemistry-mediated biotin conjugation, enriched recombinant RIPK1 kinase domains in in vitro reactions, a process inhibited by excess spermidine.
Further analysis identified K140 on RIPK1 as a critical acetylation site, with acetylation reduced ninefold in Nat1 KO MEFs. A rabbit polyclonal antibody targeting ac-hyp-K140 RIPK1 was developed and validated in RIPK1 KO 293T cells. Spermidine treatment increased ac-hyp-K140 RIPK1 levels in WT-reconstituted cells, confirming spermidine-mediated hypusination and acetylhypusination as regulatory mechanisms for RIPK1 inhibition.

In addition to the Anti-Hypusine Antibody (Hpu24) highlighted in this study, which contributed to the publication of the authors’ work in a top-tier journal, Anti-Hypusine Antibody (Hpu98) (Catalog No.: RGK08103) from AntibodySystem was recently cited in a PLOS ONE article published on February 12, 2025, titled "Development of a reliable, sensitive, and convenient assay for the discovery of new eIF5A hypusination inhibitors". This study introduced Hyp’Assay, a robust and sensitive method for detecting hypusination inhibitors. Hyp’Assay holds significant value for researchers aiming to streamline hypusine-related studies and serves as a powerful tool for large-scale chemical library screening to identify novel hypusination inhibitors.
Research Outlook
This study elucidates the intricate regulatory network among mNAT1, spermidine, and RIPK1, establishing RIPK1 as a central player in T2D pathogenesis and progression. RIPK1-mediated endothelial cell death and inflammation directly drive T2D onset, accelerate vascular complications, and contribute to end-stage renal injury. Targeting RIPK1, such as through kinase inhibitors, offers a promising avenue to improve insulin resistance and vascular health, heralding new hope for T2D patients.
Products List:
|
Catalog No. |
Product Name |
|
RGK08102 |
Anti-Hypusine Antibody (Hpu98.61) |
|
RGK08103 |
Anti-Hypusine Antibody (Hpu98) |
|
RGK08104 |
Anti-Hypusine Antibody (Hpu24.b) |
|
RGK08101 |
Anti-Hypusine antibody (Hpu24) |
|
|
