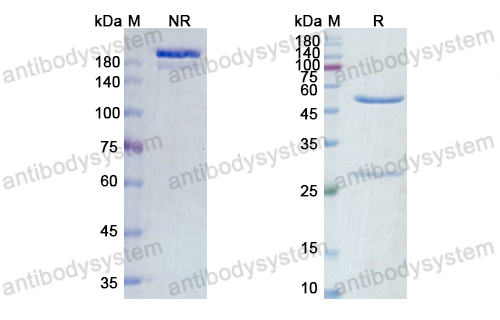
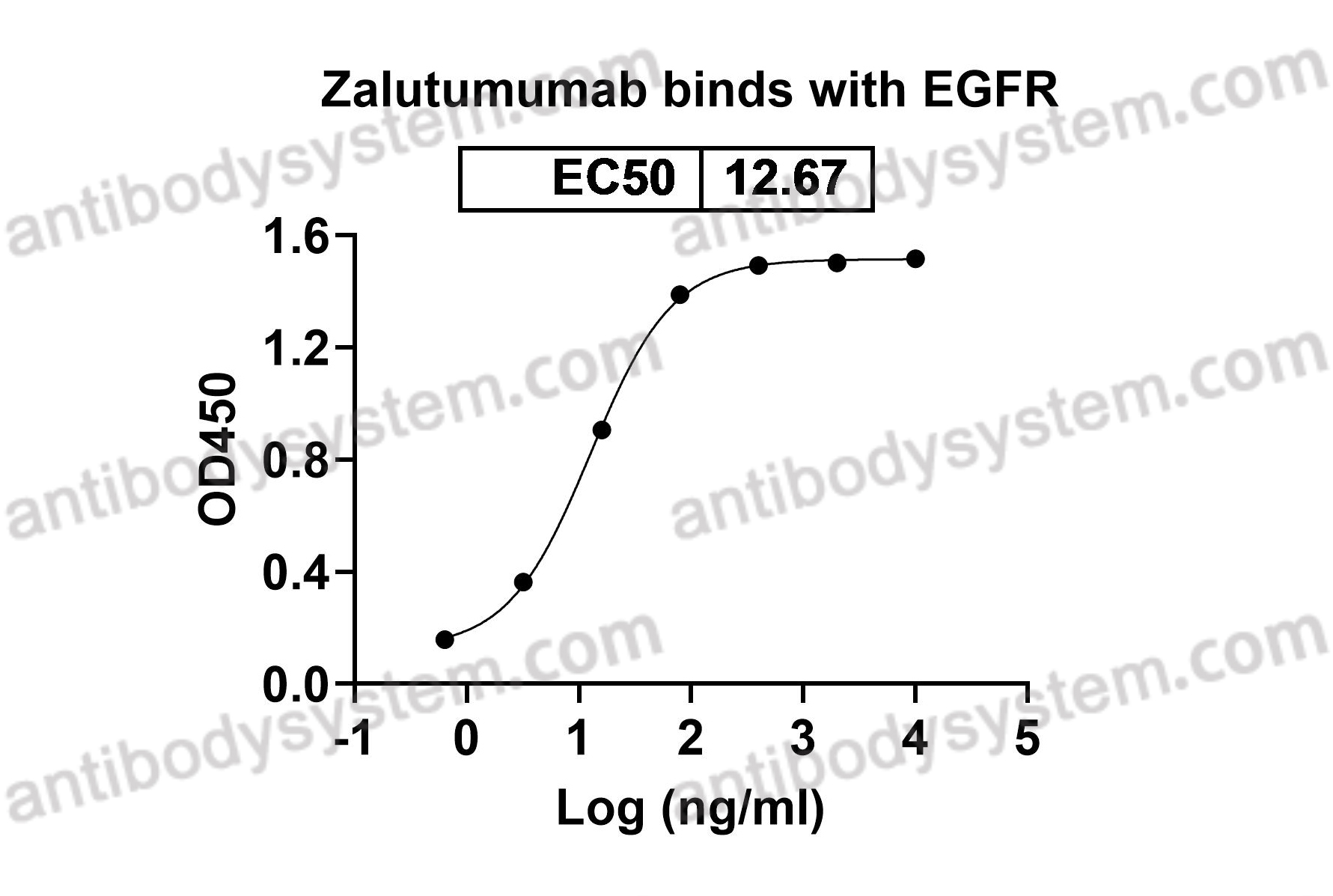
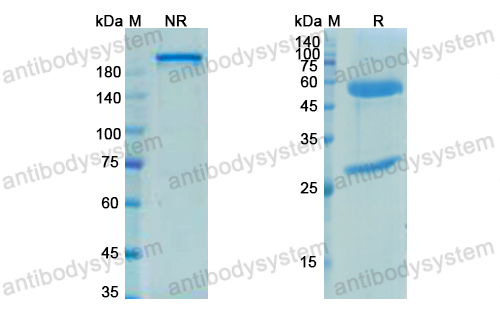
Current situation of zalutumumab, PMID: 19379120
Zalutumumab in head and neck cancer, PMID: 22171666
Retraction for Lammerts van Bueren et al. The antibody zalutumumab inhibits epidermal growth factor receptor signaling by limiting intra- and intermolecular flexibility, PMID: 22431606
Current situation of Panitumumab, Matuzumab, Nimotuzumab and Zalutumumab, PMID: 18097777
Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: an open-label, randomised phase 3 trial, PMID: 21377930
Management of recurrent and metastatic oral cavity cancer: Raising the bar a step higher, PMID: 31837576
Associations between skin rash, treatment outcome, and single nucleotide polymorphisms in head and neck cancer patients receiving the EGFR-inhibitor zalutumumab: results from the DAHANCA 19 trial, PMID: 29771169
An open-label single-arm, phase II trial of zalutumumab, a human monoclonal anti-EGFR antibody, in patients with platinum-refractory squamous cell carcinoma of the head and neck, PMID: 24714973
The antibody zalutumumab inhibits epidermal growth factor receptor signaling by limiting intra- and intermolecular flexibility, PMID: 18427122
Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage, PMID: 19949082
Molecularly targeted therapies in head and neck cancers, PMID: 23036118
Application of molecular targeted therapies in the treatment of head and neck squamous cell carcinoma, PMID: 29725456
Novel human antibody therapeutics: the age of the Umabs, PMID: 18702090
Complement-mediated tumor-specific cell lysis by antibody combinations targeting epidermal growth factor receptor (EGFR) and its variant III (EGFRvIII), PMID: 21718386
Targeted therapy in head and neck cancer, PMID: 22373581
Epidermal growth factor receptor (EGFR) antibody-induced antibody-dependent cellular cytotoxicity plays a prominent role in inhibiting tumorigenesis, even of tumor cells insensitive to EGFR signaling inhibition, PMID: 21832160
Promising new molecular targeted therapies in head and neck cancer, PMID: 23440867
Neutralization of IL-8 prevents the induction of dermatologic adverse events associated with the inhibition of epidermal growth factor receptor, PMID: 22761877
Treatment options for patients with recurrent or metastatic squamous cell carcinoma of the head and neck, who progress after platinum-based chemotherapy, PMID: 22498572
Immune-checkpoint inhibitors versus other systemic therapies in advanced head and neck cancer: a network meta-analysis, PMID: 33629592
[The current status of development of anti-EGFR antibodies], PMID: 20495305
Advances in EGFR-directed therapy in head and neck cancer, PMID: 21196389
EGFR targeting drugs in the treatment of head and neck squamous cell carcinoma, PMID: 20415599
Antibody-based therapeutics to watch in 2011, PMID: 21051951
Anti-EGFR monoclonal antibody in cancer treatment: in vitro and in vivo evidence, PMID: 21196277
Current role of EGF receptor monoclonal antibodies and tyrosine kinase inhibitors in the management of head and neck squamous cell carcinoma, PMID: 23098115
New approaches to EGFR inhibition for locally advanced or metastatic squamous cell carcinoma of the head and neck (SCCHN), PMID: 22252310
Biologic therapy in head and neck cancer: a road with hurdles, PMID: 22745915
Current challenges and clinical investigations of epidermal growth factor receptor (EGFR)- and ErbB family-targeted agents in the treatment of head and neck squamous cell carcinoma (HNSCC), PMID: 24216225
Three-dimensional histologic validation of high-resolution SPECT of antibody distributions within xenografts, PMID: 24686779
Overview of Current Treatment Options and Investigational Targeted Therapies for Locally Advanced Squamous Cell Carcinoma of the Head and Neck, PMID: 26967327
NTCP model validation method for DAHANCA patient selection of protons versus photons in head and neck cancer radiotherapy, PMID: 31432744
Epidermal growth factor receptor targeted therapy in stages III and IV head and neck cancer, PMID: 20567625
Predicting the F(ab)-mediated effect of monoclonal antibodies in vivo by combining cell-level kinetic and pharmacokinetic modelling, PMID: 22399130
Risk of fatigue in cancer patients receiving anti-EGFR monoclonal antibodies: results from a systematic review and meta-analysis of randomized controlled trial, PMID: 29181651
DAHANCA19: A randomized phase III study of primary curative (chemo)-radiotherapy and the EGFR-inhibitor zalutumumab for squamous cell carcinoma of the head and neck., PMID:40258418
Enhancing Neutrophil Cytotoxicity of a Panel of Clinical EGFR Antibodies by Fc Engineering to IgA3.0., PMID:38958494
Anti‑epidermal growth factor receptor monoclonal antibody therapy in locally advanced head and neck cancer: A systematic review of phase III clinical trials., PMID:38873325
Comparison of Second-Line Treatments for Patients with Platinum-Resistant Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: A Systematic Review and Bayesian Network Meta-Analysis., PMID:36139631
Epidermal Growth Factor Receptor as Target for Perioperative Elimination of Circulating Colorectal Cancer Cells., PMID:35035479
Immune-checkpoint inhibitors versus other systemic therapies in advanced head and neck cancer: a network meta-analysis., PMID:33629592
Management of recurrent and metastatic oral cavity cancer: Raising the bar a step higher., PMID:31837576
NTCP model validation method for DAHANCA patient selection of protons versus photons in head and neck cancer radiotherapy., PMID:31432744
Associations between skin rash, treatment outcome, and single nucleotide polymorphisms in head and neck cancer patients receiving the EGFR-inhibitor zalutumumab: results from the DAHANCA 19 trial., PMID:29771169
Application of molecular targeted therapies in the treatment of head and neck squamous cell carcinoma., PMID:29725456
Risk of fatigue in cancer patients receiving anti-EGFR monoclonal antibodies: results from a systematic review and meta-analysis of randomized controlled trial., PMID:29181651
Overview of Current Treatment Options and Investigational Targeted Therapies for Locally Advanced Squamous Cell Carcinoma of the Head and Neck., PMID:26967327
An open-label single-arm, phase II trial of zalutumumab, a human monoclonal anti-EGFR antibody, in patients with platinum-refractory squamous cell carcinoma of the head and neck., PMID:24714973
Three-dimensional histologic validation of high-resolution SPECT of antibody distributions within xenografts., PMID:24686779
Current challenges and clinical investigations of epidermal growth factor receptor (EGFR)- and ErbB family-targeted agents in the treatment of head and neck squamous cell carcinoma (HNSCC)., PMID:24216225
Promising new molecular targeted therapies in head and neck cancer., PMID:23440867
Current role of EGF receptor monoclonal antibodies and tyrosine kinase inhibitors in the management of head and neck squamous cell carcinoma., PMID:23098115
Molecularly targeted therapies in head and neck cancers., PMID:23036118
Neutralization of IL-8 prevents the induction of dermatologic adverse events associated with the inhibition of epidermal growth factor receptor., PMID:22761877
Biologic therapy in head and neck cancer: a road with hurdles., PMID:22745915
Treatment options for patients with recurrent or metastatic squamous cell carcinoma of the head and neck, who progress after platinum-based chemotherapy., PMID:22498572
Retraction for Lammerts van Bueren et al. The antibody zalutumumab inhibits epidermal growth factor receptor signaling by limiting intra- and intermolecular flexibility., PMID:22431606
Predicting the F(ab)-mediated effect of monoclonal antibodies in vivo by combining cell-level kinetic and pharmacokinetic modelling., PMID:22399130
Targeted therapy in head and neck cancer., PMID:22373581
New approaches to EGFR inhibition for locally advanced or metastatic squamous cell carcinoma of the head and neck (SCCHN)., PMID:22252310
Zalutumumab in head and neck cancer., PMID:22171666
Epidermal growth factor receptor (EGFR) antibody-induced antibody-dependent cellular cytotoxicity plays a prominent role in inhibiting tumorigenesis, even of tumor cells insensitive to EGFR signaling inhibition., PMID:21832160
Complement-mediated tumor-specific cell lysis by antibody combinations targeting epidermal growth factor receptor (EGFR) and its variant III (EGFRvIII)., PMID:21718386
Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: an open-label, randomised phase 3 trial., PMID:21377930
Advances in EGFR-directed therapy in head and neck cancer., PMID:21196389
Anti-EGFR monoclonal antibody in cancer treatment: in vitro and in vivo evidence., PMID:21196277
Antibody-based therapeutics to watch in 2011., PMID:21051951
Epidermal growth factor receptor targeted therapy in stages III and IV head and neck cancer., PMID:20567625
[The current status of development of anti-EGFR antibodies]., PMID:20495305
EGFR targeting drugs in the treatment of head and neck squamous cell carcinoma., PMID:20415599
Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage., PMID:19949082
Current situation of zalutumumab., PMID:19379120
Novel human antibody therapeutics: the age of the Umabs., PMID:18702090
The antibody zalutumumab inhibits epidermal growth factor receptor signaling by limiting intra- and intermolecular flexibility., PMID:18427122
Current situation of Panitumumab, Matuzumab, Nimotuzumab and Zalutumumab., PMID:18097777