Catalog No.
DHF92402
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Chimeric
Isotype
IgG4-lambda
Clonality
Monoclonal
Target
Tumor necrosis factor receptor superfamily member 17, TNFRSF17, CD269, B-cell maturation protein, BCMA, BCM, T3E, T-cell surface antigen T3/Leu-4 epsilon chain, CD3e, CD3E, T-cell surface glycoprotein CD3 epsilon chain
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
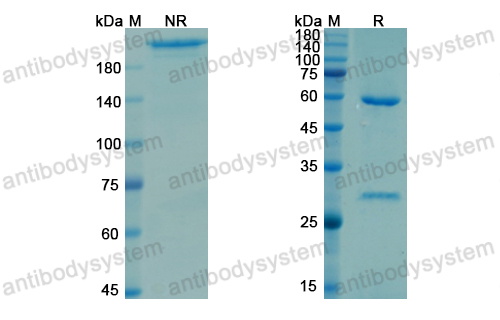
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q02223 & P07766
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
Bispecific, BCMA, JNJ-64007957, JNJ-7957, Ab-957, CAS: 2119595-80-9
Clone ID
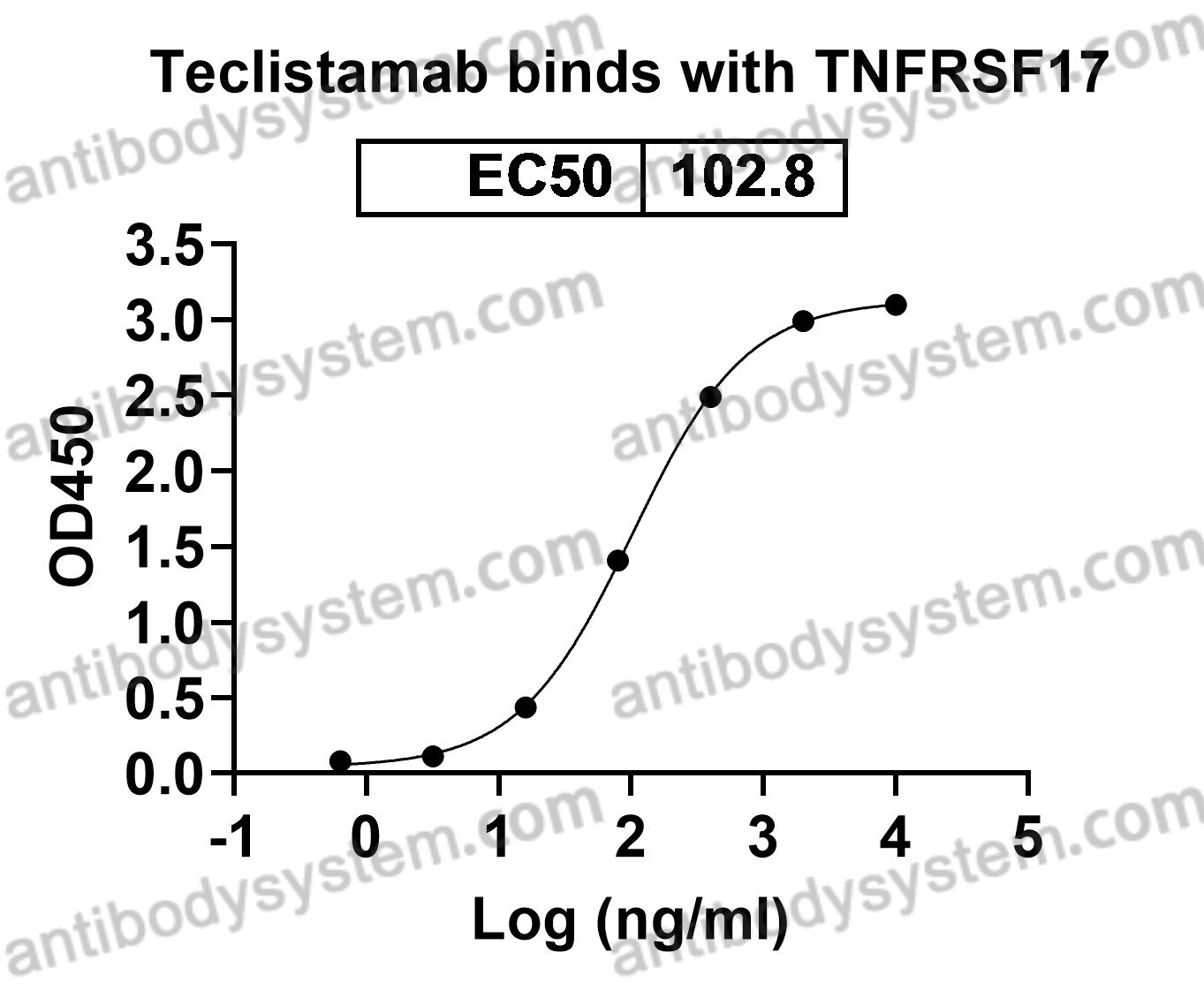
Teclistamab
Teclistamab is an active T cell-redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma, PMID: 32956453
T-cell redirecting bispecific antibodies targeting BCMA for the treatment of multiple myeloma, PMID: 33227097
MajesTEC results with teclistamab in RRMM, PMID: 34453130
Teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): a multicentre, open-label, single-arm, phase 1 study, PMID: 34388396
A BCMA-Targeted Bispecific Antibody Is Active in Multiple Myeloma, PMID: 34417227
Beyond BCL2 (B cell lymphoma) and BTK (Bruton tyrosine kinase) inhibitors: novel agents and resistance mechanisms for chronic lymphocytic leukemia., PMID:40515863
From Molecular Precision to Clinical Practice: A Comprehensive Review of Bispecific and Trispecific Antibodies in Hematologic Malignancies., PMID:40508128
Cost per responder for teclistamab and elranatamab in relapsed or refractory multiple myeloma in the United States., PMID:40495704
One-year remission of therapy-resistant SLE after a single course with the bispecific CD3: BCMA antibody teclistamab, but induction of a non-infectious Crohn's-like inflammatory bowel disease., PMID:40450406
Sequential Myelomatous Pleural and Pericardial Effusions in Multiple Myeloma: A Case Report Demonstrating Extended Survival with Teclistamab., PMID:40421459
Teclistamab-Induced Remission of Chronic Immune Thrombocytopenia in a Patient With Multiple Myeloma., PMID:40421450
Matching-Adjusted Indirect Comparison of Elranatamab versus Teclistamab in Patients with Triple-Class Exposed/Refractory Multiple Myeloma: Updated Results., PMID:40384938
Using bispecific antibodies in a patient with peritoneal dialysis for relapsed multiple myeloma., PMID:40356496
Effectiveness and safety of teclistamab for relapsed or refractory multiple myeloma: a systematic review and meta-analysis., PMID:40352937
Outcomes of elderly patients with relapsed refractory multiple myeloma (RRMM) treated with teclistamab: a multicenter study from the U.S. Multiple Myeloma Immunotherapy Consortium., PMID:40346049
Feasibility and Safety of Outpatient Model for Administration of Bispecific Antibodies: Proceedings from an International Myeloma Society 21st Annual Meeting Oral Abstract., PMID:40345961
Teclistamab Dosing in Responders: Modeling and Simulation Results from the MajesTEC-1 Study in Relapsed/Refractory Multiple Myeloma., PMID:40332715
[Immunosubstitution of patients with refractory multiple myeloma treated with teclistamab, a bispecific antibody]., PMID:40318828
Pulmonary adverse events associated with monoclonal antibodies approved for multiple myeloma: a pharmacovigilance study based on the FDA adverse event reporting system., PMID:40302414
Prolonged COVID-19 Pneumonia in Patients with Hematologic Malignancies: Clinical Significance and Serial CT Findings., PMID:40283531
Emicizumab and Acquired Hemophilia A Secondary to Multiple Myeloma., PMID:40273904
Effect of Intravenous Immunoglobulin (IVIG) Supplementation on infection-free survival in recipients of BCMA-directed bispecific antibody therapy for multiple myeloma., PMID:40268898
The first case of Teclistamab interference with serum electrophoresis and immunofixation., PMID:40241333
Outpatient Management of Bispecific Related Toxicities: An Observational Study of Safety Outcomes and Resource Utilization., PMID:40233295
Real-World Evidence Evaluating Teclistamab in Patients with Relapsed/Refractory Multiple Myeloma: A Systematic Literature Review., PMID:40227780
Economic Impact of Elranatamab for Treatment of Patients with Relapsed or Refractory Multiple Myeloma., PMID:40225701
Teclistamab versus B-cell maturation antigen-targeting chimeric antigen receptor T-cell therapy in multiple myeloma: a comparative effectiveness analysis., PMID:40207713
Real-world treatment patterns for teclistamab and talquetamab in multiple myeloma (MM): experience from 609 patients., PMID:40199861
Teclistamab for Patients with Heavily Pretreated Relapsed/Refractory Multiple Myeloma and Renal Impairment., PMID:40198766
Cost-Effectiveness of Elranatamab Versus Current Therapies for the Management of Patients with Triple-Class Exposed, Relapsed and Refractory Multiple Myeloma, Including Other Bispecific and Physician's Choice of Treatment in Spain., PMID:40193043
Characteristics of second primary malignancies following bispecific antibodies therapy., PMID:40187754
Real-world evaluation of teclistamab for the treatment of relapsed/refractory multiple myeloma (RRMM): an International Myeloma Working Group Study., PMID:40175336
Outcomes of frailty subgroups of older adults (age ≥ 70) treated with teclistamab: an International Myeloma Foundation immunotherapy database real-world analysis., PMID:40113913
Teclistamab therapy for refractory type 1 cryoglobulinemia., PMID:40109179
Unveiling cardiovascular and respiratory toxicities with monoclonal antibodies in multiple myeloma: disproportionality analysis from the FDA Adverse Event Reporting System., PMID:40095047
Treatment of Multiple Myeloma in Patients Refractory to Daratumumab/Anti-CD38 Monoclonal Antibodies: A Systematic Review., PMID:40052837
Restarting Teclistamab and Talquetamab after Prolonged Dose Delay May Not Require Re-Step-up Dosing., PMID:40048741
Teclistamab for relapsed or refractory multiple myeloma., PMID:40040734
Dexamethasone for the management of CRS Related to teclistamab in patients with relapsed/refractory multiple myeloma., PMID:40038247
Teclistamab in relapsed systemic sclerosis after autologous haematopoietic stem cell transplantation., PMID:40000264
Synergistic effect of teclistamab with PD-1 inhibition: a case of acute interstitial nephritis with dual immunotherapy., PMID:39974847
Non-tuberculous mycobacterial infections following teclistamab in multiple myeloma., PMID:39911104
Advancements in bispecific antibodies for multiple myeloma: What's new and what lies ahead., PMID:39880754
Monitoring M-Protein, Therapeutic Antibodies, and Polyclonal Antibodies in a Multiparametric Mass Spectrometry Assay Provides Insight into Therapy Response Kinetics in Patients with Multiple Myeloma., PMID:39861781
Injection site reaction to teclistamab in a patient with multiple myeloma., PMID:39845465
Expert Opinion on Multiple Myeloma Treatment in Brazil in the Bispecific Antibody Era., PMID:39809660
Incidence of Acute Kidney Injury in Relapsed and Refractory Multiple Myeloma treated with Teclistamab versus CAR T-cells., PMID:39805729
Talquetamab plus Teclistamab in Relapsed or Refractory Multiple Myeloma., PMID:39778168
Teclistamab as Successful Treatment of Relapsed TEMPI Syndrome., PMID:39774321
Real-world safety and efficacy of teclistamab in relapsed/refractory multiple myeloma: results from a multicenter, retrospective study and descriptive meta-analysis., PMID:39756041
Update on B-cell maturation antigen-directed therapies in AL amyloidosis., PMID:39748220
Practical insights into bispecific antibody therapy in multiple myeloma., PMID:39729045
Real-World Safety and Health Care Resource Utilization of Teclistamab Under an Outpatient Model for Step-Up Dosing Administration., PMID:39705632
Recurrent Cardiac Tamponade from Multiple Myeloma While Receiving Teclistamab., PMID:39691339
Comprehensive Review of Bispecific Antibody Constructs In Multiple Myeloma: Affinities, Dosing Strategies and Future Perspectives., PMID:39676006