Catalog No.
PHF19802
Species reactivity
Human
Host species
Rabbit
Isotype
IgG
Clonality
Polyclonal
Immunogen
E. coli - derived recombinant Human CLDN18/Claudin-18 (Asp28-Arg80).
Tested applications
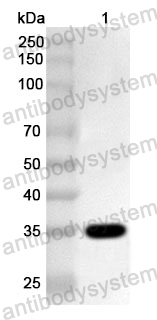
ELISA: 1:4000-1:8000, IHC: 1:50-1:100, WB: 1:1000-1:4000
Target
Claudin-18, CLDN18, UNQ778/PRO1572
Purification
Purified by antigen affinity column.
Accession
P56856
Applications
ELISA, IHC, WB
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4, 50% Glycerol, 0.05% Proclin 300.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8°C for frequent use. Store at -20 to -80°C for twelve months from the date of receipt.
Navigating the clinical challenges of zolbetuximab in patients with claudin positive advanced gastric cancer., PMID:40466467
Zolbetuximab-clzb: Targeting Claudin 18.2 in Advanced Gastric and Gastroesophageal Adenocarcinoma., PMID:40426305
Claudin 18 immunohistochemistry in cholangiocarcinoma., PMID:40386615
CLDN18.2: a potential nanotherapeutic target for cholangiocarcinoma., PMID:40206086
Targeted Radionuclide Therapy of CLDN18.2-Positive Gastric Cancer with [131I]I-Zolbetuximab: An In Vitro and In Vivo Study., PMID:40200396
Claudin18.2-positive gastric cancer-specific changes in neoadjuvant chemotherapy-driven immunosuppressive tumor microenvironment., PMID:40128286
Claudin 18 (43-14A clone) expression in pancreatic ductal adenocarcinoma: Assessment of a potential clinical biomarker for zolbetuximab therapy., PMID:40117781
Expression of Claudin18.2 in metastatic lesions in peritoneum of gastric cancer., PMID:40115911
A CLDN18.2-Targeted Nanoplatform Manipulates Magnetic Hyperthermia Spatiotemporally for Synergistic Immunotherapy in Gastric Cancer., PMID:40019387
Evaluating the pharmacokinetics of zolbetuximab in gastric adenocarcinoma., PMID:40015974
Claudin18.2 expression in gallbladder cancer correlates with immune activation and a favourable prognosis., PMID:39947883
Redefining phenotypic intratumor heterogeneity of pancreatic ductal adenocarcinoma: a bottom-up approach., PMID:39935174
The value of serum tumor-associated autoantibodies in screening and diagnosis of gastric cancer., PMID:39900126
Claudin 18.2 Immunohistochemistry Expression in Gastric Cancer: A Systematic Review., PMID:39894972
Utility of 131I-HLX58-Der for the Precision Treatment: Evaluation of a Preclinical Radio-Antibody-Drug-Conjugate Approach in Mouse Models., PMID:39839455
Claudin 18.2 Expression in 1404 Digestive Tract Adenocarcinomas Including 1175 Colorectal Carcinomas: Distinct Colorectal Carcinoma Subtypes Are Claudin 18.2 Positive., PMID:39826799
The high efficacy of claudin18.2-targeted CAR-T cell therapy in advanced pancreatic cancer with an antibody-dependent safety strategy., PMID:39797399
Zolbetuximab for Unresectable and Metastatic Gastric and Gastroesophageal Junction Adenocarcinoma: A Review of Literature., PMID:39759684
Exploring novel therapeutic targets in small bowel adenocarcinoma: insights from claudin 18.2, nectin-4, and HER3 expression analysis., PMID:39754977
Human Epidermal Growth Factor Receptor 2 Positive Advanced Gastric or Esophagogastric Adenocarcinoma: Reflecting on the Past to Gain a New Insights., PMID:39753814
Expression of claudin-18.2 in cholangiocarcinoma: a comprehensive immunohistochemical analysis from a German tertiary centre., PMID:39731204
Canadian Consensus Recommendations for Predictive Biomarker Testing in Gastric and Gastroesophageal Junction Adenocarcinoma., PMID:39727695
Efficacy and Safety of Zolbetuximab in Gastric Cancer., PMID:39699854
Expression of therapy target molecules in esophagogastric junction and Barrett's adenocarcinoma., PMID:39663311
[A New Molecular Targeted Agent for Gastric Cancer-The Anti-Claudin 18.2 Antibody, Zolbetuximab]., PMID:39648123
Emerging targets in gastric and pancreatic cancer: Focus on claudin 18.2., PMID:39637967
Relationship between [18F]FDG PET/CT findings and claudin 18.2 expression in metastatic gastric cancer., PMID:39572448
Survival outcomes of patients with gastric cancer treated with first-line nivolumab plus chemotherapy based on claudin 18.2 expression., PMID:39528778
[Claudine 18.2: A new therapeutic target in digestive cancers]., PMID:39516118
The role of CLDN18.2 in gastric cancer prognosis: a systematic review and meta-analysis., PMID:39461890
Emerging Claudin18.2-targeting Therapy for Systemic Treatment of Gastric Cancer: Seeking Nobility Amidst Danger., PMID:39364863
Targeted and combination immunotherapies using biologics for gastric cancer: the state-of-the-art., PMID:39315517
Effect of antiemetics on zolbetuximab-induced gastric injury and emesis in ferrets., PMID:39313274
Claudin 1, 4, 6 and 18 isoform 2 as targets for the treatment of cancer (Review)., PMID:39301632
CMG901, a Claudin18.2-specific antibody-drug conjugate, for the treatment of solid tumors., PMID:39232496
Enhancing antitumor efficacy of CLDN18.2-directed antibody-drug conjugates through autophagy inhibition in gastric cancer., PMID:39227365
CD64+ fibroblast-targeted vilanterol and a STING agonist augment CLDN18.2 BiTEs efficacy against pancreatic cancer by reducing desmoplasia and enriching stem-like CD8+ T cells., PMID:39187291
Targeting Claudin-18.2 for cancer therapy: updates from 2024 ASCO annual meeting., PMID:39183320
[Construction of mouse anti-human Claudin18.2 CAR-T cells and verification of in vitro functions]., PMID:39179403
The current status of immunotherapy and future horizon in the treatment of metastatic and locally advanced gastroesophageal adenocarcinoma., PMID:39171531
Variability in morphology and immunohistochemistry of Crohn's disease-associated small bowel neoplasms: implications of Claudin 18 and Cadherin 17 expression for tumor-targeted immunotherapies., PMID:39164422
[What's new in gastric cancer?]., PMID:39146748
Claudin-18.2 Immunohistochemical Evaluation in Gastric and Gastroesophageal Junction Adenocarcinomas to Direct Targeted Therapy: A Practical Approach., PMID:39098518
The impact of CLDN18.2 expression on effector cells mediating antibody-dependent cellular cytotoxicity in gastric cancer., PMID:39095563
Zolbetuximab on the SPOTLIGHT-a light spot?, PMID:38988087
Zolbetuximab: First Approval., PMID:38967717
Preparation of Radiolabeled Zolbetuximab Targeting CLDN18.2 and Its Preliminary Evaluation for Potential Clinical Applications., PMID:38949095
Synthesis, preclinical evaluation and pilot clinical translation of [68Ga]Ga-PMD22, a novel nanobody PET probe targeting CLDN18.2 of gastrointestinal cancer., PMID:38926162
The preclinical discovery and development of zolbetuximab for the treatment of gastric cancer., PMID:38919123
Extent and clinical significance of the therapy-relevant tight junction protein Claudin 18.2 in pancreatic ductal adenocarcinoma - real-world evidence., PMID:38917592