Catalog No.
KAH02201
Description
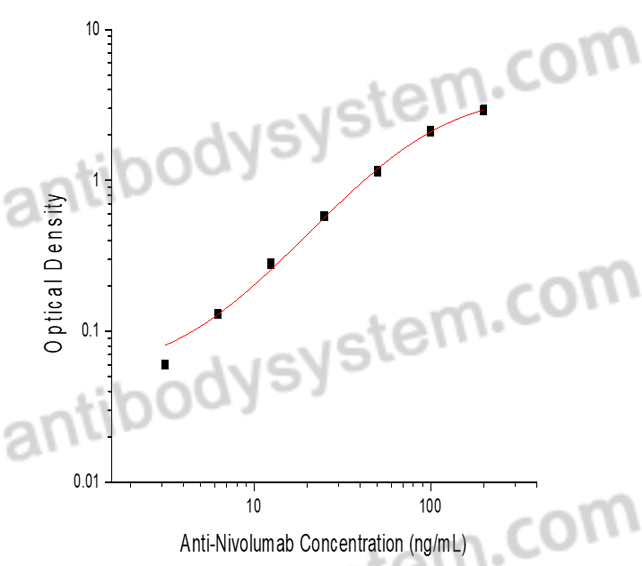
PRINCIPLE OF THE ASSAY
This assay employs the quantitative sandwich enzyme immunoassay technique. Nivolumab has been pre-coated onto a microplate. Samples or standards are pipetted into microwells and anti-Nivolumab will be captured by immobilized Nivolumab. After washing away any unbound substances, a biotin-labeled Nivolumab is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-Nivolumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-Nivolumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
3.13 - 200 ng/mL
Sensitivity
1.77 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
101.1
|
25.5
|
6.8
|
110.2
|
27.1
|
7.6
|
|
Standard deviation
|
3.8
|
0.8
|
0.6
|
4.0
|
1.3
|
0.5
|
|
CV (%)
|
3.7
|
3.3
|
8.1
|
3.6
|
4.6
|
6.7
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
BMS-936558,MDX-1106,ONO-4538,CAS: 946414-94-4
Phase II study of rucaparib and nivolumab in patients with leiomyosarcoma., PMID:40514070
Time toxicity of nivolumab in metastatic head and neck squamous cell carcinoma patients: a single-institution experience., PMID:40514043
Expanding access to cancer immunotherapy: A systematic review of low-dose PD-(L)1 inhibitor strategies., PMID:40513286
Nivolumab plus relatlimab in advanced melanoma: RELATIVITY-047 4-year update., PMID:40513285
Impaired Overall Survival of Melanoma Patients Due to Antibiotic Use Prior to Immune Checkpoint Inhibitor Therapy: Systematic Review and Meta-Analysis., PMID:40507352
Immune Checkpoint Inhibitors in the Treatment of Advanced Melanoma in Older Patients: An Overview of Published Data., PMID:40507314
Single-Center Cohort of Pediatric Patients with High-Risk Neuroblastoma Receiving Immunotherapy., PMID:40507305
Baseline Radiomics as a Prognostic Tool for Clinical Benefit from Immune Checkpoint Inhibition in Inoperable NSCLC Without Activating Mutations., PMID:40507271
Tight competition for the first line treatment of Hodgkin's lymphoma: a comprehensive review of practice-changing developments in recent years., PMID:40506833
Neoadjuvant nivolumab plus chemotherapy improves overall survival in resectable NSCLC., PMID:40506587
Combined immunotherapy with nivolumab and ipilimumab with and without sequential or concomitant stereotactic radiotherapy in patients with melanoma brain metastasis: An international retrospective study., PMID:40505525
Treatment of older patients with Hodgkin lymphoma., PMID:40504313
Case Report: Locally invasive thyroid metastases from renal cell carcinoma: surgery after neoadjuvant therapy., PMID:40502628
NEXUS: a phase I dose escalation study of selinexor plus nivolumab and ipilimumab in Asian patients with advanced/metastatic solid malignancies., PMID:40502306
Engraftment Syndrome Following Autologous Stem Cell Transplantation in a Patient Receiving Nivolumab as Salvage Therapy., PMID:40497705
Obesity paradox role in the immunosuppressive treatment of hepatocellular carcinoma., PMID:40497086
Enfortumab vedotin induced interstitial lung disease: A first case report with pathological evidence from transbronchial lung cryobiopsy., PMID:40496844
Drug-associated abdominal aortitis and retroperitoneal fibrosis after treatment with nivolumab., PMID:40496660
The real-world safety of Nivolumab: a pharmacovigilance analysis based on the FDA adverse event reporting system., PMID:40491923
Clinical Outcomes in Patients With Muscle-Invasive Urothelial Carcinoma Treated With Nivolumab., PMID:40489108
Biomarker analysis and treatment dynamics following preoperative ipilimumab plus nivolumab in locally advanced urothelial cancer from the phase 1B NABUCCO study., PMID:40489082
Implementing performance-based risk-sharing agreements in non-small cell lung cancer immunotherapy: a real-world data case study., PMID:40489036
Organ-Specific Responses to Nivolumab Plus Ipilimumab in Advanced Hepatocellular Carcinoma: A Multicenter, Retrospective Study., PMID:40488227
Signet Ring Cell Carcinoma of the Stomach with Chylous Pleural Effusion: Laterality Shift and Fluid Composition Change., PMID:40487559
Safety and short-term outcomes of adjuvant nivolumab therapy at 480 mg dose every four weeks in resected esophageal cancer: a prospective cohort study., PMID:40484886
Cost-Utility and Budget Impact Analysis of Immunotherapy for First-Line Treatment of Advanced Kidney Cancer in Latin America: Evidence From Uruguay., PMID:40482312
Validation of a 15-Gene Prognostic Signature in Metastatic Clear Cell Renal Cell Carcinoma., PMID:40479621
A multicenter Phase II study of mFOLFOX6 plus nivolumab for gastric cancer with severe peritoneal metastases: WJOG16322G., PMID:40476844
"Attenuated" Pulmonary Tumor Thrombotic Microangiopathy on Anti-Vascular Endothelial Growth Factor Treatment: A Case Report., PMID:40475293
A phase II study of nivolumab, ipilimumab, and radiation in combination with influenza vaccine in patients with pancreatic cancer (INFLUENCE)., PMID:40475164
Immune checkpoint inhibitor therapy in metastatic renal cell carcinoma: tumour response and immune-related renal vasculitis following cytoreductive nephrectomy., PMID:40474803
Cell-mediated mucositis of the oral cavity: narrative review on etiology, clinico-pathological aspects and malignant transformation., PMID:40474707
Inherited mitochondrial genetics as a predictor of immune checkpoint inhibition efficacy in melanoma., PMID:40473950
Assessing individual head and neck squamous cell carcinoma patient response to therapy through integration of functional and genomic data., PMID:40473660
Treatment-Free Survival Over 6 Years of Follow-up in Patients With Metastatic Non-small Cell Lung Cancer Treated With First-Line Nivolumab Plus Ipilimumab Versus Chemotherapy in CheckMate 227 Part 1., PMID:40473108
Phase II trial dedicated to non-selected, pretreated cutaneous angiosarcoma: Efficacy of nivolumab (AngioCheck Study)., PMID:40472568
Nivolumab Induces Durable Remission in Relapsed/Refractory ALK-Negative ALCL After Allogeneic Blood Stem Cell Transplantation-A Case Report and Literature Review., PMID:40469417
Risk factors for checkpoint inhibitor pneumonitis in lung cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis., PMID:40469302
Peripheral memory B cell population maintenance and long-term survival after perioperative chemoimmunotherapy in NSCLC (NADIM trial)., PMID:40468805
Immune-mediated enterocolitis is associated with immune checkpoint inhibitors: A pharmacovigilance study from the FDA Adverse Event Reporting System (FAERS) database., PMID:40465755
Successful use of nivolumab and relatlimab as salvage therapy for metastatic uveal melanoma., PMID:40465233
Impact of medications on the efficacy of immune checkpoint inhibitors in patients with recurrent or metastatic head and neck cancer., PMID:40465127
Personalized drug stratification using endoscopic samples to assess ex vivo gastric cancer tissue susceptibility to chemotherapy and immune checkpoint inhibitors., PMID:40465055
Malignant mesothelioma with a novel BAP1 germline frameshift mutation treated with dual immune checkpoint inhibitors: A case report., PMID:40463358
Primary Vaginal Melanoma or Clear Cell Sarcoma: A Difficult Differential Diagnosis., PMID:40462828
Claudin-18 isoform 2-specific CAR T-cell therapy (satri-cel) versus treatment of physician's choice for previously treated advanced gastric or gastro-oesophageal junction cancer (CT041-ST-01): a randomised, open-label, phase 2 trial., PMID:40460847
Neoadjuvant immunotherapy for patients with resectable stage III/IV cutaneous melanoma - A Swedish retrospective real-world study (NEO-MEL)., PMID:40456667
Cutaneous adverse effects of combination epidermal growth factor receptor inhibitor and immune checkpoint inhibitor cancer therapy., PMID:40455297
Overall Survival with Neoadjuvant Nivolumab plus Chemotherapy in Lung Cancer., PMID:40454642
Surgical Management of Destructive Thyroiditis Triggered by Neoadjuvant Immune Checkpoint Inhibitor Therapy in Locally Advanced Non-Small Cell Lung Cancer: A Case Report., PMID:40454085